Large-scale trials helped the BNT162b2 COVID-19 vaccine for full approval
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
A number of large-scale trials helped the BNT162b2 COVID-19 vaccine for full FDA approval
- Israel new drug for COVID-19: EXO-CD24 can reduce deaths by 50%
- COVID-19 vaccines for children under 12 will be available soon
- Breakthrough infection of Delta: No difference from regular COVID-19 cases
- French research: ADE occurred in Delta variant and many doubts on it
- The viral load of Delta variant is 1260 times the original COVID-19 strain
Large-scale trials helped the BNT162b2 COVID-19 vaccine for full FDA approval. A number of large-scale clinical trials helped the BNT162b2 mRNA COVID-19 vaccine obtain full FDA approval
The world is still in the pandemic of the new coronavirus pneumonia (COVID-19). Countries are racing to develop and deploy safe and effective vaccines to help prevent COVID-19.
At present, a number of new coronavirus vaccines have obtained emergency authorization (EUA) or are on the market with conditions. Recently, the BNT162b2 mRNA Covid-19 vaccine was officially approved by the U.S.
Food and Drug Administration (FDA) on August 23, 2021 local time. It is used to prevent COVID-191 for people aged 16 and above. It is the first to obtain full FDA approval. The COVID-19 vaccine is a milestone. The official and complete approval of BNT162b2 is inseparable from the support of the results of a number of large clinical trials.
The effectiveness and safety clinical trial results of multiple populations and long-term follow-up are the cornerstones of the FDA’s full approval
This time, BNT162b2 was formally approved and the application is a biological agent license application (BLA), which is compared with EUA (EUA is the FDA’s review channel for special drugs under public health emergencies. For the COVID-19 vaccine, the phase III clinical trial For more than half of the subjects to complete the follow-up for 2 months is one of the very important criteria for the COVID-19 vaccine application EUA), the amount of data needs to be more abundant, and BioNTech and Pfizer submitted about 340,000 pages of application materials for this.
The FDA analyzed the effectiveness and safety data of approximately 20,000 vaccinators aged 16 and over based on the comprehensive data package submitted. The clinical trial results showed that during the 6-month follow-up period after the 2 doses of vaccination, it prevented symptomatic infection of the new coronavirus. The effectiveness of up to 91%1, and can effectively prevent potentially serious consequences, including the risk of hospitalization and death.
In terms of safety, more than half of the vaccinators received safety follow-up at least 4 months after the second dose, and about 12,000 vaccinators were followed up for at least 6 months. The most frequently reported adverse events were pain, redness, and redness at the injection site. Swelling, fatigue, headache, muscle or joint pain, chills, and fever.
The good efficacy and safety observed within 6 months after two doses of BNT162b2 in the whole course of inoculation are the key to the formal and complete FDA approval
The 6-month follow-up data of the Phase III clinical trial submitted by the BLA application has become one of the most important evidence for this approval2.
6 months after BNT162b2 vaccination, the vaccine effectiveness (VE) of the vaccine remains above 80%, and the VE for people who have been infected with SARS-CoV-2 can reach more than 90%
The VEs from 7 days to 2 months, 2 months to 4 months, and 4 months to the data cutoff of the second dose of BNT162b2 were 96.2% (95%CI: 93.3%-98.1%) and 90.1% (95 %CI: 86.6%~92.9%), 83.7% (95%CI: 74.7%~89.9%).
Seven days after the second dose of vaccination, BNT162b2 had 91.3% (95% CI: 89.0%-93.2%) and 91.1% (95% CI: 88.8%~93.0%).
In addition, the study also confirmed that BNT162b2 can reduce the risk of severe illness in subjects after neocoronavirus infection. After the first dose and 7 days after the second dose, BNT162b2 can prevent severe VE of 96.7% (95%CI: 80.3%~99.9%) and 95.7% (95%CI: 73.9%~99.9%), respectively.
BNT162b2 is well tolerated 6 months after vaccination, which is similar to the safety of 2 months after vaccination
The safety of BNT162b2 6 months after vaccination is similar to the safety data observed in the previously published phase III clinical trials of vaccination with 2 doses and 2 months follow-up3. The local adverse reactions and systemic adverse reactions of BNT162b2 were mainly mild to moderate. There were no grade 4 adverse reactions, and no cases of myocarditis were found. During the 6-month follow-up period, no serious or unresolvable or incurable adverse events were found.
Multi-country real-world studies further confirm the effectiveness and safety of BNT162b2 after full vaccination
U.S.
The health systems of many states in the United States have counted 51,795 real-world evidence of vaccinators, showing that the VE of BNT162b2 for prevention of new coronavirus infection, COVID-19-related hospitalization, and intensive care unit (ICU) were 86.1% (95%CI: 82.4%~ 89.1%), 88.8% (95%CI: 75.5%~95.7%), 100% (95%CI: 51.4%~100%), suggesting that BNT162b2 can reduce the rate of new coronavirus infection and reduce the impact of COVID-19 on the healthcare system Burden 4.
Israel
The Israeli National Mass Vaccination Study conducted follow-up observations of 596,618 vaccinators after 2 doses and found that 7 days or more after the second dose, the VE for recorded new coronavirus infections (including symptomatic and asymptomatic) was 92% (95%CI: 88%~95%), the VE for symptomatic COVID-19 infection is 94% (95%CI: 87%~98%), and the VE for hospitalization is 87% (95%CI: 55%) ~100%), the VE for severe cases is 92% (95%CI: 75%~100%)5.
Canada
Canadian real-world studies show that 7 days after the second dose of BNT162b2 vaccination, the corrected VE for symptomatic infections caused by Alpha, Beta/Gamma, and Delta mutant strains are 89% (95% CI: 86%~91%) and 84%, respectively (95%CI: 69%~92%), 87% (95%CI: 64%~95%); correction VE respectively for serious prognosis (hospitalization or death) caused by Alpha, Beta/Gamma, and Delta mutant strains It is 95% (95%CI: 92%~97%), 95% (95%CI: 81%~99%)6.
Other countries
The results of a large-scale negative case-control study in the United Kingdom revealed that the preventive effect of two doses of BNT162b2 on the Delta variant strain was 88.0%7. A study on the effectiveness of vaccination against nearly 20,000 COVID-19 cases in Scotland shows that the effective rate of preventing symptomatic COVID-19 infection caused by the Delta variant 14 days after vaccination with 2 doses of BNT162b2 is 83%8.
The latest booster immunization results of the third dose show that BNT162b2 can increase the body’s neutralizing antibody titer and respond to the invasion of mutant strains
Vaccine immunity may weaken over time. Timely vaccination of the third dose of booster can further increase the neutralizing antibody titers in the body and enhance the body’s immunity to deal with the invasion of Delta mutant strains and other unforeseen mutant strains.
The results of the booster immunization study of the third dose of BNT162b2 showed that for the original and Beta mutant strains, the neutralizing antibody titer after the third dose was much higher than that of the second dose, increasing by 5 to 8 times and 15 to 21 times, respectively (Figure A) ; Against the currently widespread Delta variant, after the third dose of vaccine, neutralizing antibody titers increased by 5 times and 11 times in adults aged 18 to 55 and elderly people aged 65 to 80, respectively (Figure B). According to calculation and comparison, compared with the third dose before vaccination, the neutralizing antibody titer of the Delta mutant strain of the third dose of booster immunization increased to close to the level of neutralizing antibody against the original strain, and the neutralization effect is estimated to increase by 100 times.
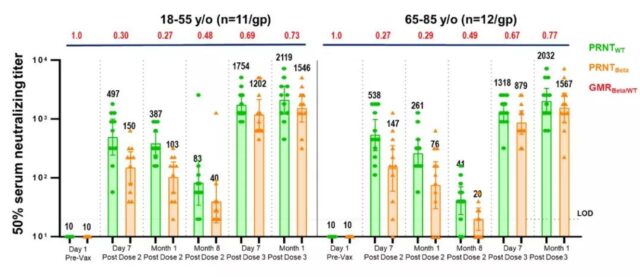
A: Neutralizing antibody titers against the original strain and Beta variant strain after inoculation of the third dose
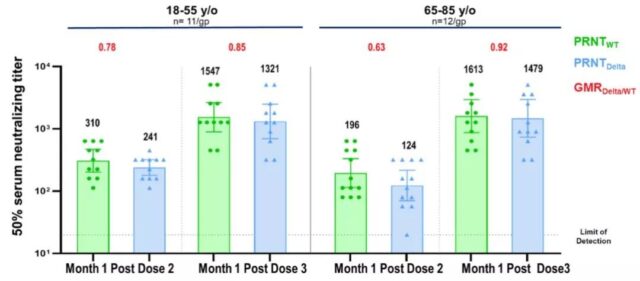
B: Neutralizing antibody titers against the original strain and the Delta mutant strain after the third dose of inoculation
In response to the FDA’s full approval of BNT162b2, Peter Marks, MD, Director of the FDA’s Center for Biologics Evaluation and Research, said: “Our scientific and medical experts have conducted a very thorough and thoughtful evaluation of this vaccine. We have evaluated hundreds of thousands of pages of science. Data and information, analyzed the safety and effectiveness of BNT162b2, and conducted a detailed evaluation of the production process, including inspections of production facilities. Although the FDA quickly approved this vaccine, it is fully in line with the existing United States High standards for vaccines.”
BNT162b2 can also provide a third dose of vaccine for adolescents aged 12 to 15 and certain immune-compromised groups according to EUA. Since December 2020, more than 1 billion doses of BNT162b2 have been inoculated in more than 120 countries or regions around the world, and its effectiveness and safety in preventing COVID-19 infection have been internationally recognized. In the future, it will be planned to supplement the BLA to obtain the permission of the third dose or booster for people 16 years and older, and to obtain the full approval of the FDA for the use of people 12 to 15 years old.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



