Roche SERD therapy Giredestrant Eoadjuvant treatment of ER+/HER2
- FDA Approved Opdualag: The First Immunotherapy Targeting LAG-3
- Cutaneous Reactions Following COVID-19 Vaccination: A Review of the Evidence
- Brief Intermittent Exercise Reduces Heart Disease and Death Risk
- Personalized Lung Tumor Chips Assess PD-1 Therapy Response
- Study Shows Prior Infection Offers Strong Immunity to Original COVID-19 Strain
- Chinese Food Products Dominate Korean Tables Amid Safety Concerns
Roche SERD therapy giredestrant neoadjuvant treatment of ER+/HER2- early postmenopausal breast cancer: significantly inhibit tumor proliferation!
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Roche SERD therapy Giredestrant Eoadjuvant treatment of ER+/HER2.
New breast cancer drug! Roche SERD therapy giredestrant neoadjuvant treatment of ER+/HER2- early postmenopausal breast cancer: significantly inhibit tumor proliferation!
Compared with anastrozole, giredestrant treatment significantly reduced Ki67, which is a prognostic marker of tumor proliferation.
Roche recently announced the interim data of the randomized Phase 2 coopERA breast cancer trial at the 2021 European Society of Medical Oncology (ESMO) virtual conference.
The trial is evaluating the next generation of oral selective estrogen receptor degrading agent (SERD) giredestrant (formerly known as GDC-9545) neoadjuvant treatment of ER-positive, HER2-negative postmenopausal early breast cancer (eBC) patients.
The results show that:
In the window of opportunity after 14 days of treatment, compared with anastrozole, giredestrant treatment showed a decrease in Ki67, a prognostic marker for tumor proliferation (80% vs 67%, p=0.0222, respectively). The safety of giredestrant is consistent with previous trials, and fewer patients have side effects related to giredestrant and anastrozole.
Levi Garraway, MD, Roche’s Chief Medical Officer and Head of Global Product Development, said: “We are very happy to share with you the first random phase 2 data of Giredestrant, showing that Giredestrant is very interesting in early HR-positive and HER2-negative breast cancer patients. Encouraging activity and safety.
Our ongoing HR-positive breast cancer comprehensive project aims to address the significant unmet needs of patients who are still experiencing profound impacts on their quality of life, including drug resistance and the risk of disease recurrence. “
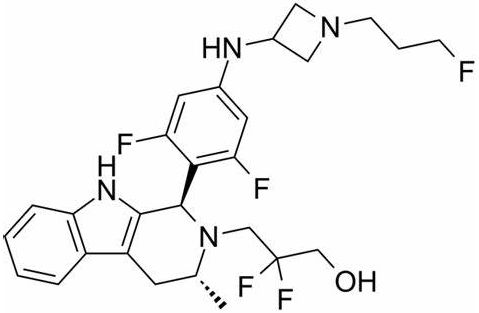 giredestrant chemical structure (picture source: medchemexpress.com)
giredestrant chemical structure (picture source: medchemexpress.com)
Giredestrant is an oral, new-generation SERD designed to completely block the ER signal. The drug has a strong receptor occupancy (Receptor Occupancy) and shows special preclinical characteristics. Estrogen promotes the growth of HR-positive breast cancer cells by attaching to the ER.
Giredestrant blocks the receptor to prevent the effects of estrogen, and in the process causes the receptor to degrade. In clinical trials, giredestrant has shown efficacy regardless of ESR1 mutation status (ESR1 gene mutation is an important mechanism of hormone therapy resistance).
In December 2020, the US FDA granted giredestrant Fast Track Qualification (FTD) for the treatment of ER-positive, HER2-negative, second-line and third-line metastatic breast cancer.
giredestrant is administered orally and has encouraging clinical efficacy and safety, and has shown superior efficacy to other SERDs before clinical use.
Oral giredestrant has the potential to change the treatment experience of patients. Compared with intramuscular injection, it provides a treatment option with greater convenience and less pain.
Interim analysis data from 83/202 patients enrolled in the coopERA breast cancer study showed that compared with anastrozole, giredestrant has better antiproliferative activity and has good safety in HR-positive and HER2-negative breast cancer :
During the window of opportunity (1-14 days) for this neoadjuvant (preoperative) study, the relative Ki67 reduction was used as a proliferation biomarker to evaluate the pharmacodynamic effect of giredestrant, which indicates the ability of the treatment to inhibit tumor growth:
(1) Ki67 of giredestrant was reduced by an average of 80% (95%CI: -85%, -72%), while Ki67 of anastrozole was reduced by an average of 67% (95%CI; 95%CI: -75%, -56%) ), p=0.0222.
(2) In patients with baseline Ki67 ≥ 20% or baseline Ki67 <20%, consistent Ki67 inhibition was observed with giredestrant treatment (baseline Ki67 ≥ 20% population: 83% vs 71%; baseline Ki67 <20% population Medium: 65% vs 24%).
(3) After 14 days of treatment, 25% of the tumors in the giredestrant treatment group showed a complete cell cycle arrest rate (CCCA), and anastrozole was 5% (Δ20%; 95% CI: -37% and -3%).
The safety of giredestrant is consistent with its mechanism of action, and the side effects associated with giredestrant (28%) are less than that of anastrozole (38%). There were no adverse events (AE) or serious adverse events ≥ grade 3 related to giredestrant.
Roche SERD therapy Giredestrant Eoadjuvant treatment of ER+/HER2.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.