Trial results of mRNA vaccines for children aged 5-11 are finally released
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Trial results of mRNA vaccines for children aged 5-11 are finally released
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Trial results of mRNA vaccines for children aged 5-11 are finally released.
The results of clinical trials of mRNA vaccines for children aged 5-11 are finally here! Parents of elementary school students can’t wait for a long time.
On September 20, Pfizer-BioNTech’s BNT162b2 mRNA vaccine demonstrated very good safety and effectiveness in a Phase 2/3 clinical trial in children aged 5-11.
Whether or not children should be vaccinated has made parents quite entangled.
It also makes us have to repeatedly evaluate the three basic points of whether to vaccinate: the necessity of vaccination, the effectiveness of the vaccine, and the safety of the vaccine.
The need for vaccination
The necessity for adults to be vaccinated against COVID-19 is beyond doubt, and the fatality rate is too high.
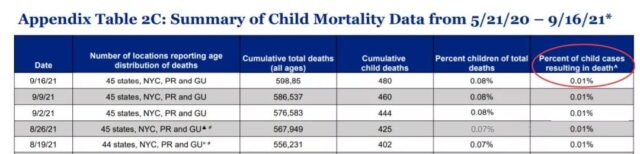
(The case fatality rate of children with COVID-19 in the United States. Source: American Academy of Children)
However, children are different. According to the latest data released by the American Academy of Children (AAP) on September 16, 2021, the fatality rate for children from COVID-19 in the United States is 0.01%, which is slightly higher than that caused by influenza.
In the UK, in the first year of the COVID-19 pandemic, 25 children died, most of them with serious underlying diseases.
However, the biggest difference between COVID-19 and influenza is the extremely high rate of severe illness.
0.9% of COVID-19 patients in the United States have developed severe illness and require hospitalization. AAP is deeply concerned about this because the long-term prognosis of COVID-19 in children is unknown.
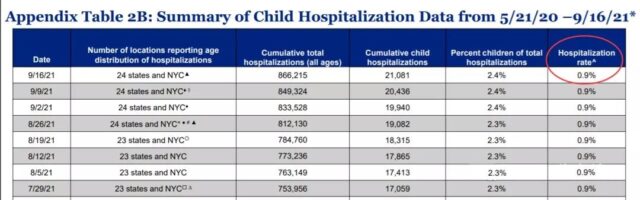
(Covid-19 hospitalization rate for children in the United States. Source: American Academy of Children)
The most influential factor in our decision-making is the infection rate of children with COVID-19.
If we look at April 2020, COVID-19 in children in the United States accounts for only 2% of all cases, a total of 9,259 children. When the possibility of infection in children is extremely low, it is true that there is no rush to vaccinate.
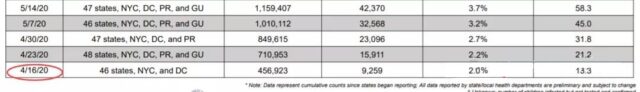
(Source: American Society of Children)
But as of September 16, 2021, the number of children COVID-19 cases in the United States has exceeded 5.51 million, accounting for 15.7% of all cases and 7.3% of all children!
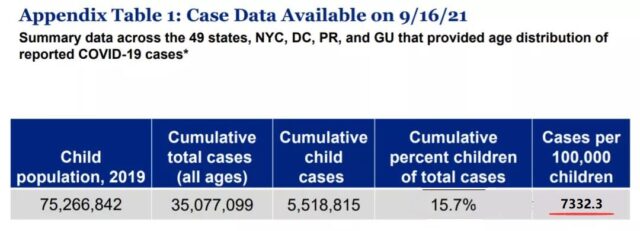 (Overview of COVID-19 in children in the United States. Source: American Academy of Children)
(Overview of COVID-19 in children in the United States. Source: American Academy of Children)
At the same time, after the beginning of the fall in the United States, almost all schools canceled the option of online courses and asked to return to school.
The daily “email notification received” (meaning there is a new diagnosis in the class), “received phone notification” (meaning close contact with the child) and other news broke out in the parent group every day, making parents anxious .
Although due to the situation, parents are eager for their children to be vaccinated earlier, the effectiveness and safety of the vaccine for children aged 5-11 years cannot be ignored.
Therefore, we carefully reviewed the clinical trial data of BNT162b2 in children aged 5-11 years.
Experimental population:
A total of 2,268 children aged 5-11 (primary school students) participated in this phase 2/3 clinical trial; after randomization, 2/3 subjects were included in the vaccination group and 1/3 subjects were included in the control group ( Placebo with saline).
Vaccination plan:
Two doses of 10ug, 21 days apart.
It can be seen that the vaccination dose for the primary school age group is only 1/3 of the adult vaccination dose.
The vaccination dose is much lower, will it affect the effectiveness?
Effectiveness:
One month after the second dose of the vaccination group, the induced geometric mean titer (GMT) of neutralizing antibodies was 1,197.6 (95% CI 1106.1-1296.6); it was equivalent to 1146.5 of GMT for 30ug vaccinators in the 16-25 age group .
The results showed that with a dose of 10ug, BNT162b2 also induced extremely high antibody levels.
Pfizer’s press release explains the reason: the 10ug dose is carefully selected as the preferred dose for safety, tolerability and immunogenicity in children aged 5-11.
Safety:
According to the information on Pfizer’s official website, subjects aged 5-11 years old have a good tolerance for COVID-19, and their discomforts are consistent with those in the 16-25 year old age group.
The vice president of Pfizer and pediatrician Dr. Gruber, who is in charge of the project, revealed: In fact, after the second dose, we saw that children aged 5-11 experienced less discomfort compared with those aged 16-25 years. .
There are currently 28 million children aged 5-11 in the United States, accounting for 9% of the total population of the United States. According to CNN, a quarter of the parents of children aged 5-11 said that once the vaccine is approved, they will vaccinate their children immediately.
According to the US Centers for Disease Control and Prevention (CDC), 57% of children aged 12-17 in the United States have received at least one dose of the COVID-19 vaccine, and 46% are fully vaccinated.
Well, it seems that the vaccine for children aged 5-11 is safe and effective.
The next question is: when can children aged 5-11 be vaccinated?
According to the information provided by Dr. Fauci, children aged 5-11 will be vaccinated before Halloween this year (October 31).
This time is more reliable, because the FDA usually spends 4-6 weeks reviewing the submitted data.
The FDA Acting Commissioner Dr. Janet Woodcock also mentioned in a statement this month that the data for young children will be reviewed as soon as possible and may be completed within a few weeks.
Delta mutant strain causes a surge in COVID-19 cases in children
The Delta mutant strain is extremely contagious and spreads very fast, which brings great difficulties to epidemic prevention; it poses a new challenge especially for children’s epidemic prevention.
In the past week, the number of new child cases in the United States accounted for 25.7% of all new cases, and 225,000 new child cases were reported in one week.
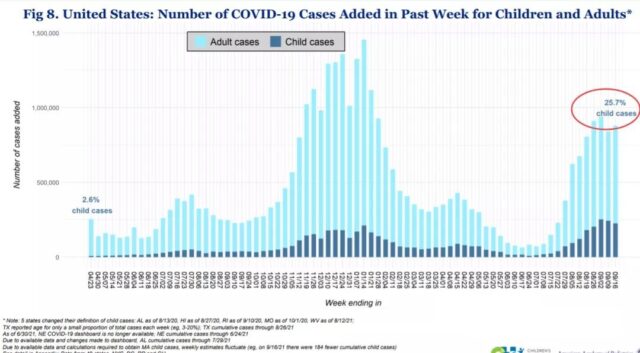 (U.S. children with COVID-19 accounted for the proportion of new cases. Source: American Academy of Children)
(U.S. children with COVID-19 accounted for the proportion of new cases. Source: American Academy of Children)
Summary:
The Phase 2/3 clinical trial of BNT162b2 mRNA vaccine for children aged 5-11 years showed that two doses of 10ug can induce extremely high titers of neutralizing antibodies with good safety.
At the same time, due to the prevalence of Delta mutant strains, the chance of children being infected has soared.
It is time to seriously consider vaccinating children.
Reference source:
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-positive-topline-results
https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



