Development of vaccines against COVID-19 Omicron is being accelerated
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
Development of vaccines against COVID-19 Omicron is being accelerated
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Development of vaccines against COVID-19 Omicron is being accelerated.
Race against the COVID-19 mutant strain: Pfizer expects a new vaccine for one hundred days, and Moderna launches three levels
The new variant strain of the COVID-19 virus has raised concerns about the protective efficacy of the COVID-19 vaccine.
On November 26, the World Health Organization named the new coronavirus variant B.1.1.529 after the Greek letter “Omicron” and officially listed it as a new coronavirus variant that “needs attention”.
Preliminary studies have shown that the omicron mutant strain increases the risk of reinfecting the human body with the virus.
The outside world is generally worried that the mutant strain may cause a new wave of ferocious epidemics after the delta strain, which will affect the effectiveness of the COVID-19 vaccination.
The WHO emphasized on November 26 that, based on scientists’ research on the genetic sequence of the new strain, “it will take several weeks to understand the impact of this variant on any vaccines under development”.
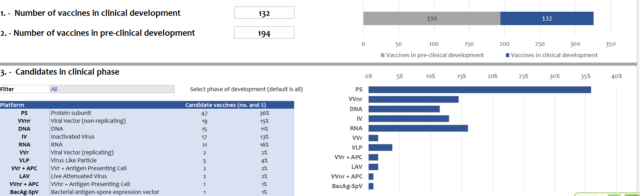
World Health Organization official website on global COVID-19 vaccine research and development data
The Paper reporters inquired on the official website of the World Health Organization and learned that as of November 26, there were a total of 362 COVID-19 vaccine candidates worldwide, of which 132 were in clinical practice.
Judging from public information, Pfizer/BioNtech, Moderna, Johnson & Johnson, AstraZeneca, Novavax, etc. are currently at the forefront of COVID-19 vaccine research and development, and have achieved commercial vaccination in different countries.
Regarding the omicron variant, the above-mentioned COVID-19 vaccine manufacturers have stated on November 26 local time that they are studying the impact of the virus strain on the vaccine, and some manufacturers have begun to respond.
Perhaps affected by the above news, US vaccine stocks rose sharply. As of the close of the day, Moderna rose more than 20%, Pfizer rose more than 6%, Biotech rose more than 14%, and Novavax rose nearly 9%.
Pfizer: Vaccines against variants can be developed and produced within 100 days
According to Fox Business Network and other foreign media reports, on November 26, local time, BioNtech (BNTX) and Pfizer (NYSE: PFE) stated that they had initiated the evaluation of the effectiveness of the mRNA COVID-19 vaccine on the omicron variant, and the latest two Results will be issued within a week.
Pfizer said: “As always, we will continue to follow science as we research the best way to protect people from the new coronavirus.
If there is a vaccine escape variant, the two companies expect to be able to develop and produce a vaccine against the virus in about 100 days. Variant specific vaccines, but need to obtain regulatory approval.”
It is worth mentioning that in June of this year, Pfizer CEO Albert said publicly: “So far, the data shows that the existing mutant strains have not escaped the protection provided by our vaccine.
We have established a process that can Develop a new vaccine when needed within 100 days.”
Biotech emphasized that the newly discovered variant is significantly different from the previously observed variant because it has additional mutations in the spike protein.
It is expected that more data from laboratory tests will be available within two weeks at the latest.
These data will provide more information about the variant strain and whether adjustments to existing vaccines are needed.
Moderna: Three levels are advancing at the same time
On November 26, local time, Moderna (Moderna, mRNA) announced a solution strategy for the B.1.1.529 (Omicron) variant, and said that the company is working quickly to test the current vaccine dose to neutralize the omicron variant. It is expected Data will be provided in the coming weeks.
Moderna’s 50 micrograms of mRNA-1273 has been authorized for emergency use in the United States for adults aged 18 and above, and received emergency use authorization for the booster needle on November 19, local time.
Regarding the mutant strain, in fact, as early as the beginning of 2021, Moderna had proposed a number of clinical strategies to deal with the delta virus mutant strain that caused global attention at that time.
Regarding the current Omicron variant, Moderna emphasized that if the currently authorized booster dose of 50 micrograms of mRNA-1273 proves to be insufficient to enhance immunity against the omicron variant, the company’s response strategy includes three levels.
First, Moderna has tested the safety and immunogenicity of higher doses of mRNA-1273 (100 micrograms) enhancers in healthy adults.
At present, the study has completed the administration of 306 participants. In addition, the National Institutes of Health (NIH) also studied mRNA-1273 at a dose of 100 micrograms, and the results showed that it produced the highest neutralizing titer against the previous new coronavirus strain.
Moderna is working to rapidly test serum from its high-dose booster recipients in a neutralization trial to determine whether the 100 microgram dose of the vaccine provides superior protection against Omicron.
Second, Moderna has studied two multivalent booster candidate vaccines, including mRNA-1273.211 and mRNA-1273.213, which are designed to predict mutations, such as those found in Omicron variants.
The former has completed the administration of mRNA-1273.211 potential critical safety and immunogenicity studies at the dose levels of 50 micrograms (N=300) and 100 micrograms (N=584); the latter has completed 100 micrograms (N=584) ) Dosing at the dose level, and plans to explore the 50 microgram dose level in approximately 584 participants.
Third, Moderna will quickly advance the candidate product mRNA-1273.529 for Omicron variants. According to Moderna, the product candidate is part of the company’s strategy to promote product candidate products for specific variants.
The company has repeatedly demonstrated its ability to advance new product candidates into clinical trials within 60-90 days.
American Moderna CEO Stephane Bancel said: “From the beginning, we have said that in the process of seeking to overcome the pandemic, we must be proactive with the development of the virus.
Omicron variant The mutation is worrying. For several days, we have been implementing our strategy as soon as possible to solve this variant.”
Johnson & Johnson: is testing the effectiveness of the COVID-19 vaccine against new variants
Johnson & Johnson (JNJ.US) is developing a COVID-19 vaccine that is an adenovirus vector technology route and is a single-dose vaccination.
The vaccine has been fully approved in Canada, and the vaccine has been authorized for emergency use of booster shots in the United States.
Regarding the response to the variant strain, according to Forbes Business Network news, Johnson & Johnson responded in the email that the company is testing the effectiveness of its COVID-19 vaccine against the B.1.1.529 variant.
A spokesperson wrote: “We are closely monitoring the emerging omicron variants and are already testing the effectiveness of their vaccines against the rapidly spreading new variants first discovered in Southern Africa.”
AstraZeneca: Studying the effect of variant strains on vaccine and antibody combination drugs
In response to the COVID-19 pneumonia epidemic, AstraZeneca (AZN.US), in addition to vaccines, also has antibody combination drugs.
The former belongs to the technical route of adenovirus vectors, and the latter is a combination of two long-acting antibodies tixagevimab (AZD8895) and cilgavimab (AZD1061).
On October 11, local time, AstraZeneca announced that the results of a phase III clinical trial showed that compared with placebo, mild non-hospital patients received an intramuscular injection of 600 mg of the antibody combination drug, and the risk of severe illness or death was reduced by 50%.
Regarding the latest variant of the new coronavirus, according to Global News, AstraZeneca said on November 26 local time that it is studying the impact of the new coronavirus variant that is spreading rapidly in South Africa on its new coronavirus vaccine and antibody combination drugs.
AstraZeneca said the company is conducting research in two southern African countries, Botswana and Swatini, to collect data, which will enable the company to collect real-world data on this mutant strain.
In addition, AstraZeneca also stated that the company has developed a vaccine platform in cooperation with Oxford University to quickly respond to new variants.
The vaccine platform was created at Oxford University. The company has previously stated that it is developing a variant vaccine to better target Beta variants.
Novavax: has begun to study a version of the COVID-19 vaccine
Novavax (NVAX) said on November 26 that it has begun working on a version of its COVID-19 vaccine to target the variants detected in South Africa and will be ready for testing and testing in the next few weeks. Production.
The COVID-19 vaccine being developed by Novavax is a protein vaccine. It has obtained emergency use authorization in Indonesia, the Philippines and other countries, and has also submitted regulatory documents in the United Kingdom, Australia, New Zealand, Canada and other countries.
In addition, Novavax has also submitted an emergency use list (EUL) to the World Health Organization (WHO), and is expected to submit complete information to the US FDA before the end of the year.
Development of vaccines against COVID-19 Omicron is being accelerated
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



