What are the differences between the current COVID-19 vaccines?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
What are the differences between the current COVID-19 vaccines?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What are the differences between the current COVID-19 vaccines? Scientists use data to tell you the truth!
The US Food and Drug Administration approved the emergency use of mRNA vaccines (Pfizer Biotech vaccine and Moderna vaccine) in December 2020, and approved the use of Johnson & Johnson single-dose vaccine in February 2021.
To date, nearly 200 million Americans have been vaccinated against COVID-19. As some people have been vaccinated over time, questions have been raised about the long-term effectiveness of the vaccine.
Previous studies have shown that Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273) and Johnson & Johnson (Ad26.COV2.S) vaccines have a powerful effect on the prevention of COVID-19.
Recently, a research team at Beth Israel Deaconess Medical Center (BIDMC) compared the immune response caused by three vaccines within 8 months.
Researchers reported the comparison of immune response and cellular immune response caused by two doses of Pfizer vaccine (31 participants), two doses of Moderna vaccine (22 participants) and a single dose of Johnson & Johnson vaccine (8 participants); evaluation; 2 to 4 weeks after the second immunization (peak period of immunity) for those who received mRNA vaccines (Pfizer Biotech vaccine and Moderna vaccine are both mRNA vaccines), and antibodies within 8 months after the first immunization of Johnson & Johnson vaccine recipients Reacts with T cells.
The article was published in The New England Journal of Medicine on October 15 and is titled “Differential Kinetics of Immune Responses Elicited by Covid-19 Vaccines”.

Research data
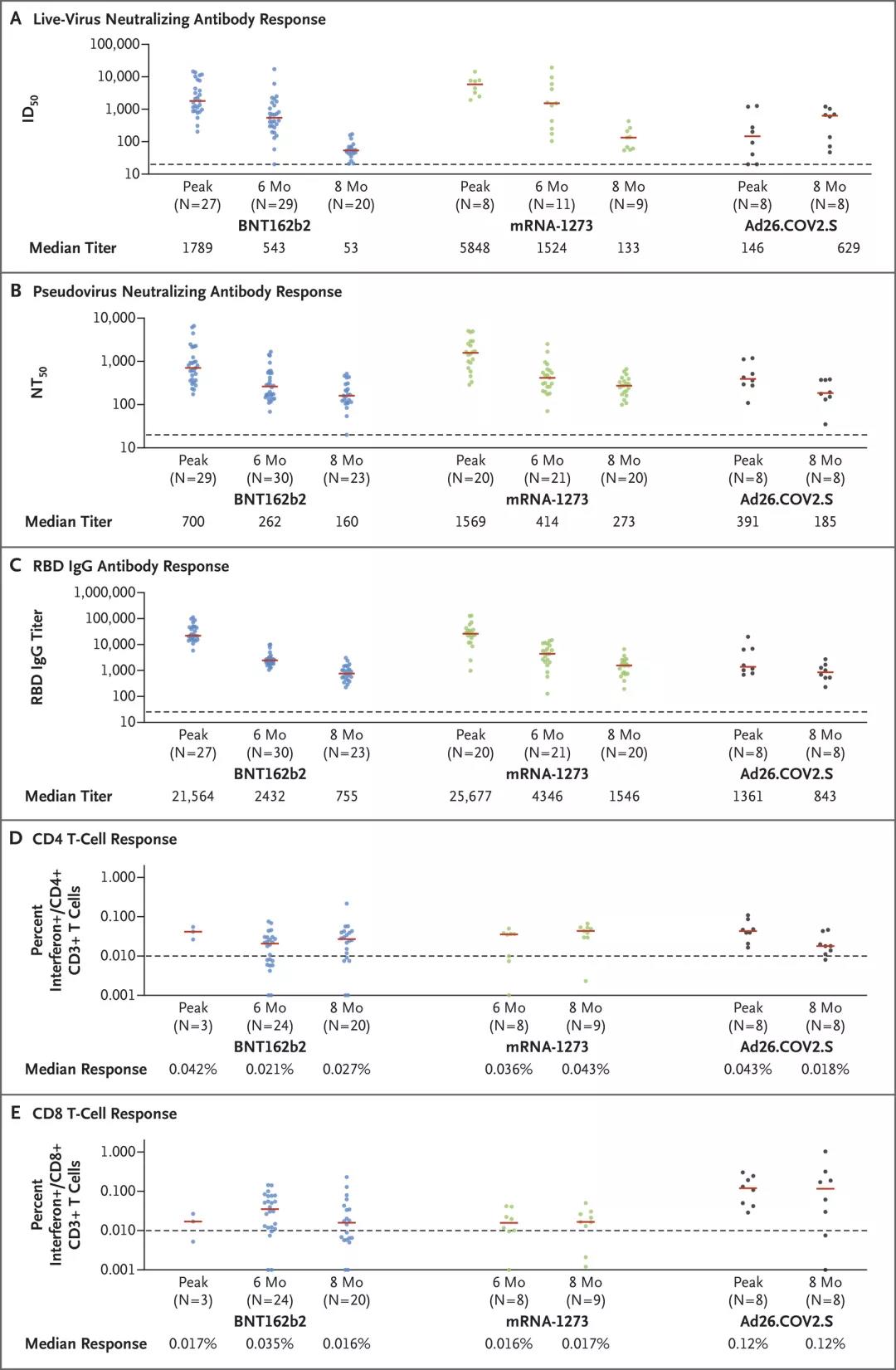
During the peak period of immunization, the median of the highest level of neutralizing antibody response to live virus caused by Pfizer vaccine was 1789, the median of the highest level of neutralizing antibody response to pseudovirus was 700, and the median of the highest level of RBD IgG antibody response was 21564.
However, as shown in the figure below, these antibody responses dropped sharply within 6 months after vaccination, and further declined within 8 months.
Eight months after Pfizer vaccination, the median of live virus neutralizing antibody response caused by the vaccine was 53, the median of pseudovirus neutralizing antibody response was 160, and the median of RBD IgG antibody response was 755, respectively. The peak period of immunity is 34 times, 4 times, and 29 times lower.
Also during the peak period of immunization, the median of the highest level of neutralizing antibody response to live virus caused by Moderna vaccine was 5848, the median of the highest level of neutralizing antibody response to pseudovirus was 1569, and the median of the highest level of RBD IgG antibody response Is 25677.
8 months after vaccination with Moderna vaccine, the median of live virus neutralizing antibody response was 133, the median of pseudovirus neutralizing antibody response was 273, and the median of RBD IgG antibody response was 1546. These antibody responses were 44 times, 6 times, and 17 times lower than the peak period of immunity.
During the peak period of immunization, the Johnson & Johnson vaccine has a significantly lower median antibody response than mRNA vaccines (Pfizer Biotech Vaccine and Moderna Vaccine).
Within 4 weeks after receiving a single dose of Johnson & Johnson vaccine, the median of live virus neutralizing antibody response was 146, the median of pseudovirus neutralizing antibody response was 391, and the median of RBD IgG antibody response was 1361; however, , These antibody responses remained relatively stable within 8 months.
At 8 months, the median of live virus neutralizing antibody responses was 629, the median of pseudovirus neutralizing antibody responses was 185, and the median of RBD IgG antibody responses was 843; these antibody responses were the same as in week 4. The time is not much different.
All three vaccines usually have stable antibody-dependent cell-mediated phagocytosis and antibody-dependent complement deposition.
After being vaccinated with Pfizer and Moderna vaccines, the live virus neutralizing antibody, pseudovirus neutralizing antibody, and the response of the vaccinated person to the SARS-CoV-2 variant RBD and spike protein specific binding antibody continued to decline from the peak period of immunity 8 months; however, after vaccination with Johnson & Johnson vaccine, the vaccinators’ antibody response to these variants is relatively stable, and even increases in some cases.
By 8 months, the median of the pseudovirus neutralizing antibody response of the three vaccines against the Delta variant (SARS-CoV-2 B.1.617.2) was similar, with Pfizer vaccine being 67, Moderna vaccine being 76, Johnson & Johnson The vaccine is 107.
T cell response was assessed by CD4+ and CD8+ intracellular cytokine staining, using mixed S-peptide for stimulation. At the 8th month, the median CD8+ T cell response of the Pfizer vaccine was 0.016%, the Moderna vaccine was 0.017%, and the Johnson & Johnson vaccine was 0.12%. In all three vaccines, T cell responses showed extensive cross-reactivity to SARS-CoV-2 variants.
Summarize
These data show the difference in immune response caused by mRNA vaccine and Johnson & Johnson vaccine within 8 months after vaccination. As shown in the above studies, the initial antibody response caused by mRNA vaccine is usually higher. The antibody response caused by Moderna vaccine is usually higher and longer than that of Pfizer vaccine, but the antibody response will continue to decline within 8 months after vaccination. The initial antibody response caused by the Johnson & Johnson vaccine was low, but these responses were relatively stable within 8 months after vaccination, with almost no signs of decline. All three vaccines showed extensive cross-reactivity to SARS-CoV-2 variants. These findings have important implications for understanding how vaccine immunity diminishes over time.
Ai-ris Y. Collier, a maternal-fetal medicine expert at BIDMC and the lead author of this article, said, “Despite the decline in neutralizing antibody levels, the stable T cell response and non-neutralizing antibody function can explain how vaccines continue to provide strong protection. Vaccines (even during pregnancy) are still our best way to end the COVID-19 pandemic.”
Reference:
https://www.nejm.org/doi/10.1056/NEJMc2115596
What are the differences between the current COVID-19 vaccines?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



