Insulin-resistant adipocytes can promote breast cancer metastasis through exosomes
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Insulin-resistant adipocytes can promote breast cancer metastasis through exosomes
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Insulin-resistant adipocytes can promote breast cancer metastasis through exosomes .
Literature title : Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes
Significance
Obesity, insulin resistance and type 2 diabetes (T2D) are risk factors for breast cancer in postmenopausal women.
Studies have shown that metabolic and inflammatory complications in obese people are closely related to the occurrence and development of estrogen receptor (ER) -positive breast cancer. Related.
However, as of now, the cellular and molecular pathways through which obesity or metabolic abnormalities mediate the incidence, progression and metastasis of breast cancer have not been fully elucidated.
In this study, the researchers compared and analyzed the differences in the tumor microenvironment of obese patients, revealing another important mechanism that has not previously been discovered to promote breast cancer progression, that is , exosomes produced by metabolically abnormal fat cells affect breast cancer cells. Potential promotion .
At the same time, in the future, this adipocyte-derived circulating exosomes can also be used as non-invasive liquid biopsy biomarkers to assist clinical decision-making in breast cancer patients at risk of progression.
Guide
Obesity, insulin resistance and type 2 diabetes (T2D) are risk factors for breast cancer in postmenopausal women.
Studies have shown that metabolic and inflammatory complications in obese people are closely related to the occurrence and development of estrogen receptor (ER) -positive breast cancer. Related.
In addition, group cohort studies have also confirmed that obesity-driven T2D is associated with the incidence, progression and mortality of breast cancer.
However, as of now, the cellular and molecular pathways through which obesity or metabolic abnormalities mediate the incidence, progression and metastasis of breast cancer have not been fully elucidated.
Tumor microenvironment (TME) plays an important role in promoting tumor progression, and adipocytes are the main non-malignant cell type in breast cancer TME.
However, compared with immune infiltrating cells, adipocytes are still lacking in research on the regulation of cancer progression.
Recently, the research team of Boston University School of Medicine published a research article titled Adipocyte-derived exosomes may promote breast cancer progression in type 2 diabetes in Science Signaling .
In this study, the differences in TME of obese patients were revealed by comparative analysis.
Another important mechanism that has not been discovered before to promote the progression of breast cancer is the potential promotion of exosomes produced by metabolically abnormal fat cells on breast cancer cells .

Image source: Science Signaling
Main research contents
Microenvironment model of adipocyte-tumor cell co-culture
In order to study the role of adipocytes in breast cancer progression, they first used the transwell system to co-culture human ER-positive cells with human adipocytes.
The results found the expression of key genes essential for epithelial cell-to-mesenchymal transition (EMT) , which are significantly related to tumor progression, such as SNAI1, SNAI2, VIM, CDH2, TWIST1, etc.
If fat cells are exposed to low-dose TNF-a and insulin resistance (Insulin-Resistant, IR) occurs for the first time , the expression of EMT-related genes will change even more.
The enrichment analysis of differentially expressed gene pathways showed that IR-type adipocytes induce EMT pathway more strongly than insulin-sensitive (IS) adipocytes in a co-culture environment .
Since TME exosomes are related to EMT, their subsequent experimental results also showed that the exosomes of undifferentiated pre-adipocyte control cannot induce EMT genes.
In summary, these results indicate that if the adipocytes are of type IR or come from patients with obesity-related metabolic complications, the exosomes derived from them will transmit more dangerous signals and promote breast cancer cells .
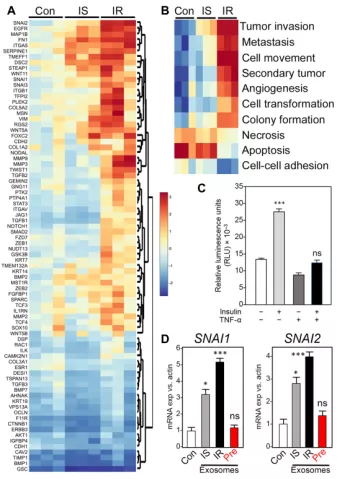 Image source: Science Signaling
Image source: Science Signaling
Adipocyte exosomes encode metabolic status
In postmenopausal obese women, estrogen production induced by adipokines and adipocytes, free fatty acids released by pro-inflammatory macrophages and adipocytes are all related to the progression of ER+ breast tumors.
Next, they compared exosomes purified from primary T2D-derived adipocytes with exosomes purified from adipocytes in a non-diabetic control group matched with age, sex, and body mass index.
The experimental results are consistent with the exosomes of IR adipocytes. Compared with the exosomes of the non-diabetic control group, the exosomes of T2D adipocytes induced high expression of genes and pathways related to breast cancer invasion.
In addition, immunofluorescence The imaging results also confirmed the differential regulation of key EMT proteins.
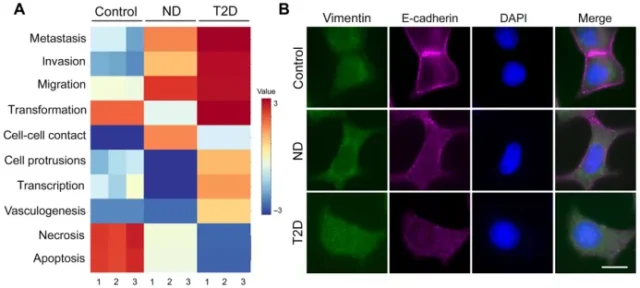
Image source: Science Signaling
The tumor-promoting characteristics of IR adipocyte-derived exosomes
The image analysis results of the human cell model processed by exosomes showed that the characteristic morphology of breast tumor cells also changed along with the changes in transcription and protein expression.
Compared with the control group, after treatment with exosomes from T2D adipocytes, cell circumference increased, elongation and roundness decreased.
It was also found that T2D exosomes promoted cell migration.
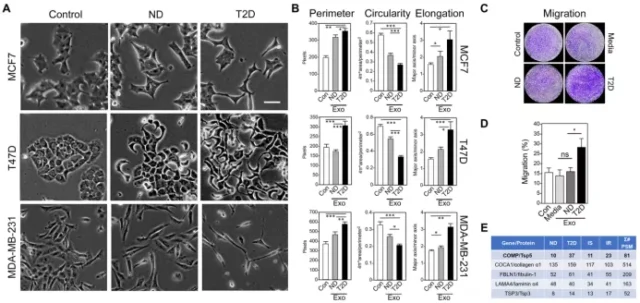 Image source: Science Signaling
Image source: Science Signaling
The results of the analysis of the difference between the control group and the T2D type of exosomal protein profiles show that thromboreactive protein 5 (TSP5) is the most significant one, and previous studies have also shown that it is related to cancer progression. Therefore, TSP5 exerts an effectual function on exosomes. May also be crucial .
To verify the role of TSP5, they used lentivirus to overexpress TSP5 in human primary preadipocytes, then differentiated the cells into adipocytes, and purified exosomes from the conditioned medium.
As expected, TSP5-rich exosomes significantly increased the transcription of ZEB1 and SNAI2 genes compared to the control group.
At the same time, immunofluorescence results showed that exosomes loaded with TSP5 indeed delivered their payload to tumor cells and induced the expression of vimentin.
In addition, exosomes released from primary adipocytes transduced by TSP5 lentivirus induced transcriptional changes related to EMT function, while control exosomes did not induce the expected transcriptional changes.
Consistent with the previous results, the results of pathway enrichment analysis of TSP5-loaded exosomes showed that the signaling pathways related to invasion, migration, and metastasis were significantly increased, while the signaling pathways related to cell death were significantly down-regulated.
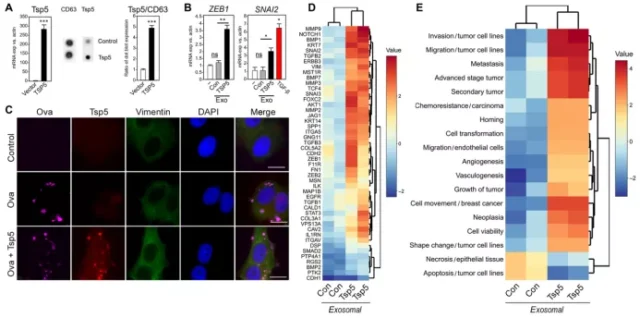 Image source: Science Signaling
Image source: Science Signaling
Co-expression of TSP5 and BET protein coding genes shortened breast cancer survival without distant metastasis
Finally, they tested the Distant Metastasis–Free Survival (DMFS) of breast cancer patients with high or low expression of COMP . COMP is the gene encoding TSP5.
They analyzed the effect of high or low level co-expression of COMP with each of the three BET encoding genes BRD2, BRD3, and BRD4 on DMFS.
Consistent with the expected results, the co-expression of COMP and higher BET genes was associated with a significant reduction in DMFS, with the strongest effect on BRD2.
Specifically, the high co-expression of BRD2 and COMP increased the risk of distant metastasis by 35% within 25 years; the high co-expression of BRD3 and COMP increased the risk by 19%.
Taken together, these findings reaffirm the importance of the function of BET genes in all 3 individual cells in obesity-related cancers, not just BRD4 (BRD4 is generally considered the only important player) .
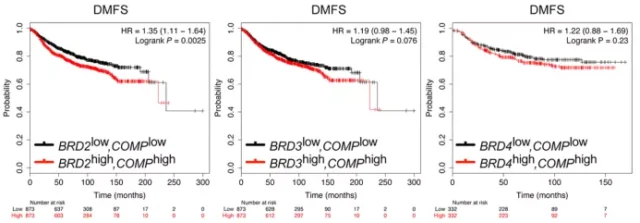 Image source: Science Signaling
Image source: Science Signaling
Conclusion:
In summary, the results of this study show that the exosomal proteins from IR adipocytes are related to the characteristics of EMT and cancer stem cells .
This result has also been confirmed in a cohort of breast cancer patients with long-term follow-up.
It is also consistent with the findings that patient metabolism is related to the incidence and risk of breast cancer progression.
In addition, in the future, circulating exosomes derived from adipocytes may be suitable as biomarkers for non-invasive liquid biopsy to assist clinical decision-making in breast cancer patients at risk of progression.
Reference:
https://www.science.org/doi/10.1126/scisignal.abj2807
Insulin-resistant adipocytes can promote breast cancer metastasis through exosomes
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



