Cell: Necessary to overcome the dysfunction of CAR T cell therapy first
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Cell: Necessary to overcome the dysfunction of CAR T cell therapy first
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Cell: Necessary to overcome the dysfunction of CAR T cell therapy first.
Chimeric antigen receptor T (CAR T) cell therapy has achieved remarkable success in hematological malignancies, but there is still no hope in the treatment of solid tumors.
Some recent studies have shown that CAR T cells have failed in the microenvironment of solid tumors. Or dysfunction is the main obstacle to the success of CAR T cell therapy for solid tumors [1] .
However, what mediates the dysfunction of CAR T cells? There is no clue yet.
In order to understand the dysfunction clues of mesothelin-redirected CAR T (M5CAR T) cells, and develop CAR T cell therapy for the treatment of solid tumors.
On December 2, 2021, the research team (M. Young, Shelley L. Berger and CAR T-cell therapy pioneer Carl H. June) from University of Pennsylvania Regina jointly published a research paper, entitled “An NK-like CAR T cell transition in CAR” in Cell. T cell dysfunction”
In this research paper, the author established an in vitro model of continuous antigen exposure by simulating CAR T cells in the tumor microenvironment, and determined the mechanism of CAR T cell dysfunction.
It is equivalent to the transformation of cells from T cells to NK-like T cells. The identification of the characteristic transcription factors ID3 and SOX4 that cause cell dysfunction has brought new hope for CAR T treatment of solid tumors.

The first step in solving this problem is to develop an in vitro model CAR T cells, the authors first established a stable continuous exposure to the antigen (the Continuous the Antigen Exposure, CAE ) model to drive CAR T-cell failure / dysfunction, RNA Sequencing results showed that the model reproduced the hallmarks of T cell failure, which is consistent with the characteristics of T cell failure/dysfunction in humans and mice.
IPA pathway analysis shows that in this model, T cell failure, PD-1/PD-L1 immunotherapy, and CTLA4 signaling pathway are enriched (Figure 1, blue) ; pathways related to natural killer (NK) cells are also enriched Set (picture 1, red) .
Multiple NK receptors are up-regulated, including KLRC1, KLRC2, KLRC3, KLRB1, KLRD1 and KIR2DL4.
Next, the author anchored several important transcription factors, including the up-regulated EGR1, ID3, SOX4, RBPJ and down-regulated KLF2, BCL6, LEF1, etc. in the CAE model.
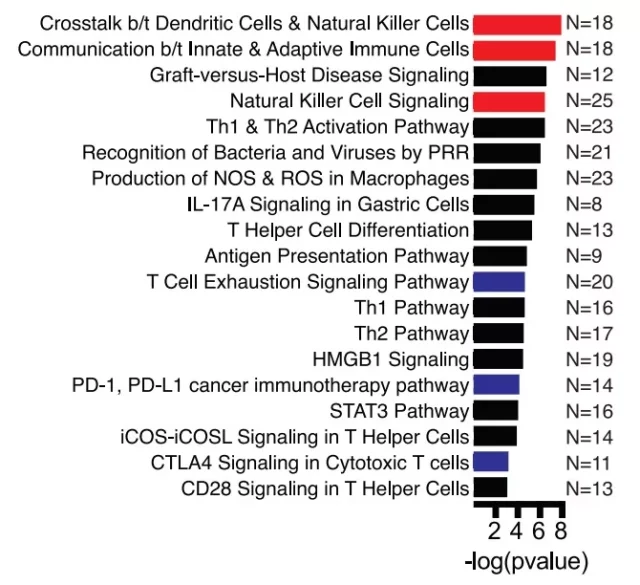
Figure 1: IPA analysis of significantly differentially expressed genes on day 28 and day 0 of CAE (red means NK, blue means cell failure pathway).
The more important breakthrough of this study is that the author also discovered a new feature of CAR T cell dysfunction in this model: the conversion of CD8+ T cells to NK-like T cells .
In order to confirm whether NK-like CAR T cell phenotypic transition also occurs in the body, the authors verified it in pancreatic tumors and lung tumors in xenotransplanted mice, and performed a retrospective evaluation of a clinical trial (NCT02030834) in cancer patients. , Identified CAR T cells with NK-like transitions.
The next breakthrough is to find a common transcription factor that regulates CAR T dysfunction and NK-like T cell transformation.
The author analyzed the single-cell RNA sequencing data set and found that ID3 and SOX4 are always co-expressed with other dysfunction characteristic genes in CAE T cells (Figure 2) .
ID3 and SOX4 are also key transcription factors in the development of memory CD8+ T cells [2] .
ID3 does not directly bind to DNA, but is a member of the helix-loop-helix transcription factor family [3] that inhibits other transcription factors from binding DNA .
Therefore, ID3 lacks a specific DNA binding motif. However, SOX4 happens to have a known DNA motif [4] . After persistent antigen exposure, CAR T cells form chromatin openings at the SOX4 site.
In order to confirm the regulation of ID3 and SOX4, the authors performed continuous antigen exposure on CAR T cells of ID3-KO and SOX4-KO for 20-28 days, and then analyzed their transcription profiles and cytotoxicity.
Unlike wild-type CAR T cells, ID3-KO and SOX4-KO CAR T cells retain anti-tumor immunity.
Although the authors did not perform in vivo experiments here to confirm whether these two transcription factors have similar functions in vivo, cell experiments suggest the plasticity of CAR T cells.
Downregulation of ID3 and SOX4 genes can improve CAR T cell dysfunction by preventing or delaying CAR T cell dysfunction. The efficacy of CAR T cell therapy for solid tumors.
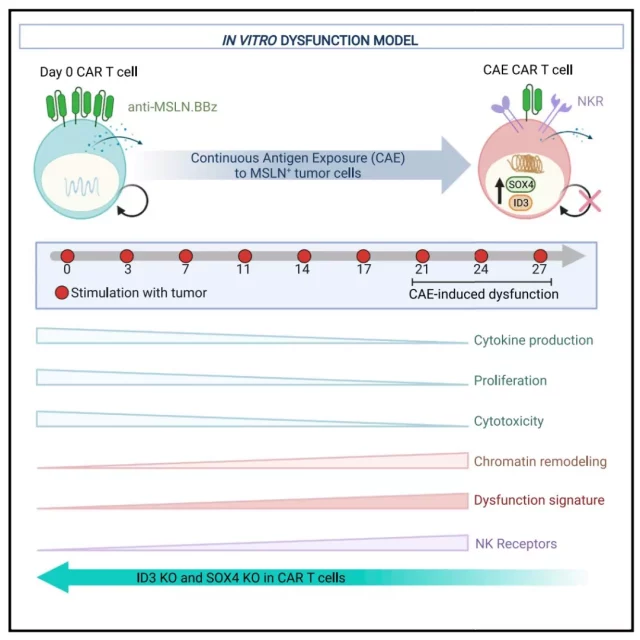
Figure 3: NK-like CAR-T cell transformation in CAR-T cell dysfunction (MSLN: Mesothelin).
In summary, the in vitro model constructed by the authors reproduced CAR T cell dysfunction and discovered a new mechanism, that is, the characteristics of the transition from CD8+ T cells to NK-like T cells (Figure 3) .
Whether in the mouse model of CAR T dysfunction, or in patients treated with CAR T cells, it has been confirmed in vivo and in vitro.
The authors further confirmed that destroying the transcription factors ID3 and SOX4 in CAR T cells can enhance tumor killing effects.
This research has led to a new strategy to improve the efficacy of CAR T cell therapy in solid tumors.
Original link:
https://doi.org/10.1016/j.cell.2021.11.016
References
1. Poorebrahim, M., et al. (2021). Counteracting CAR T cell dysfunction. Oncogene 40, 421–435.
2. Yang, CY, et al. (2011). The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat. Immunol. 12, 1221–1229.
3. Benezra, R., Davis, RL, Lockshon, D., Turner, DL, and Weintraub, H. (1990). The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61, 49 -59.
4. Fornes, O., et al. (2020). JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 48 (D1), D87–D92.
Cell: Necessary to overcome the dysfunction of CAR T cell therapy first.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



