What is the difference between adult and childhood Diffuse gliomas?
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
- Mifepristone: A Safe and Effective Abortion Option Amidst Controversy
- Asbestos Detected in Buildings Damaged in Ukraine: Analyzed by Japanese Company
What is the difference between adult and childhood Diffuse gliomas?
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
What is the difference between adult and childhood Diffuse gliomas?
The new version of the tumor classification introduces the concept of childhood-type diffuse low-grade glioma for the first time, which is characterized by histological appearance as diffuse low-grade glioma, mainly in children, but also in adults, usually with a history of epilepsy.
Molecular variants are divided into MYB or MYBL1 variants and mitogen-activated protein kinase (MAPK) pathway variants.
With the development of pathology and the advancement of pathological detection technology, especially the improvement of omics technologies such as second-generation sequencing (NGS) and whole genome methylation sequencing (WGBS), the genetic background and mechanism of tumor occurrence and development have gradually become clear.
More and more molecular biomarkers have been confirmed to play an important role in the classification, classification, grading, treatment and prognosis of central nervous system (CNS) tumors.
In 2016, the fourth revised edition of the World Health Organization (WHO) classification of central nervous system tumors (hereinafter referred to as the fourth revised edition) introduced molecular phenotypes on the basis of histological morphology for the first time, and proposed the concept of integrated diagnosis, aiming to improve the pathological The objectivity and repeatability of diagnosis, improve the individual management process, promote the development of clinical trials, basic experiments and epidemiological research, and provide support for optimizing resource allocation and formulating policies.
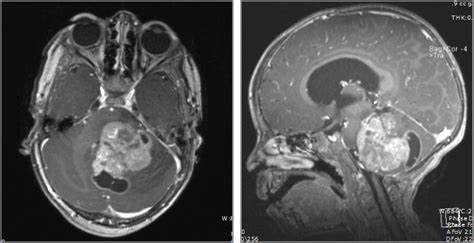
Adult diffuse glioma
IDH mutation is an important diagnostic marker for adult-type diffuse glioma.
The most common IDH mutation in gliomas is IDH1 gene codon 132 mutation (R132H mutation is the most common, others include R132C, R132S, R132G and R132L, etc.), and the rest are IDH2 gene codon 172 mutation (including R172G) , R172M and R172W, etc.) and other rare codon mutations (such as IDH1R49, etc.).
IDH-mutated diffuse glioma with 1p/19q co-deletion is diagnosed as oligodendroglioma, IDH-mutated and 1p/19q co-deletion type;
among them, TERT promoter mutation, NOTCH1 mutation, FUBP1 mutation and CIC Mutations are a common molecular feature of this type of glioma;
diffuse gliomas with IDH mutations but without 1p/19q co-deletion are diagnosed as astrocytoma, IDH mutant; ATRX mutations, TP53 mutations are this type The typical molecular variation of glioma is also an important auxiliary diagnostic marker;
and CDKN2A/B homozygous deletion is a grading marker for this type of tumor, and IDH-mutant astrocytoma with CDKN2A/B deletion is diagnosed as star Shape cell tumor, IDH mutant, CNSWHO grade 4. IDH wild-type diffuse glioma with histological appearance of necrosis or microvascular proliferation is diagnosed as glioblastoma, IDH wild-type;
IDH wild-type diffuse astrocytes with histological appearance of CNSWHO grade 2 or 3 If there is EGFR amplification, chromosome 7 amplification/chromosome 10 deletion (+7/-10), TERT promoter mutation, one of the above three molecular variants can also be diagnosed as glioblastoma, IDH wild-type, and these three molecular variants are also molecular biomarkers related to the prognosis of such tumors.
Childhood-type diffuse low-grade glioma
The new version of the tumor classification introduces the concept of childhood-type diffuse low-grade glioma for the first time, which is characterized by histological appearance as diffuse low-grade glioma, mainly in children, but also in adults, usually with a history of epilepsy.
Molecular variants are divided into MYB or MYBL1 variants and mitogen-activated protein kinase (MAPK) pathway variants.
(1) MYB or MYBL1 variant:
MYB is one of the genes in the MYB/SANT domain-containing transcription factor family, which plays an important role in controlling the proliferation and differentiation of hematopoietic and other progenitor cells, and acts as a proto-oncogene in leukemia and solid tumors.
The MYBL1 gene works similarly. MYB or MYBL1 variant forms include gene copy number variation and gene fusion (MYB partner genes include QKI, ESR1, MMP16, MAML2, PCDHGA1, etc., MYBL1 partner genes include RAD51B, MAML2, ZFHX4, TOX, etc.).
Although studies have shown that low-grade gliomas with MYB or MYBL1 mutations have similar DNA methylome patterns, it remains to be further confirmed by multi-center large-sample studies.
The new tumor classification combines histological morphology and molecular features to classify MYB or MYBL1 variant tumors into two types: diffuse astrocytoma, MYB or MYBL1 variant, and angiocentric gliomas (common with MYB-QKI fusions).
(2) MAPK pathway variant:
MAPK signal transduction pathway is an important signal transduction system of eukaryotic cells.
It transduces extracellular signals through tertiary kinase cascades and participates in various physiological processes such as cell growth, differentiation, and apoptosis. , and is closely related to the development of tumors.
Glioma MAPK pathway-related gene variants include NF1, BRAF, FGFR1, CRAF, NTRK, PTPN11, ROS1, etc.
Molecular variants of pleomorphic low-grade neuroepithelial tumor in young adults (PLNTY) include BRAFV600E, FGFR3-TACC3 fusion, FGFR2-CTNNA3 fusion, and FGFR2-KIAA1598 fusion.
In diffuse low-grade gliomas, common molecular variants of MAPK pathway variants include FGFR1 tyrosine kinase domain (TKD) repeats, FGFR1 mutations, FGFR1 fusions, and BRAFV600E mutations, BRAF fusions, and BRAF insertion mutations [18].
Due to the lack of specificity of some molecular mutations in MAPK pathway variant tumors, the classic pathological diagnosis methods and results such as histological morphology and immunohistochemical staining are very important.
Childhood Diffuse High-Grade Glioma
(1) Histone H3 variant:
H3 is one of the five major histones involved in eukaryotic chromatin structure, and its sequence variation and modification state play an important role in the dynamic and long-term regulation of genes.
The new version of the tumor classification adds diffuse midline glioma, H3K27 variant, to further expand the definition of diffuse midline glioma.
According to molecular variation, it is divided into 3 subtypes, namely H3K27 mutant (including H3K27M and H3K27I), EGFR mutant (mostly involving the thalamus), and H3 wild with EZHIP overexpression.
H3K27 mutation occurs in H3.3 (encoding gene H3F3A) and H3.1 (encoding gene HIST1H3B/C), and the mutation rate of H3F3A is about 3 times that of HIST1H3B/C, and the prognosis is worse.
Molecular variants such as TP53 mutation, ACVR1 mutation, PPM1D mutation, and PDGFRA amplification are common molecular genetic features of H3K27-mutant diffuse midline glioma.
Another tumor carrying histone H3 mutation is diffuse hemispheric glioma, H3G34 mutation, which mainly occurs in the cerebral hemisphere, showing that the 34th glycine (Gly) of histone H3.3 is replaced by arginine (Arg) or valine.
Amino acid (Val) substitution missense mutation (H3.3G34R/V). Glioma H3.3G34 mutation mainly occurs in the H3F3A gene, often accompanied by ATRX mutation and TP53 mutation.
(2) H3 wild type and IDH wild type:
diffuse childhood high-grade glioma, H3 wild type and IDH wild type are more common in children and young adults, and have high-grade tumor histological features, but the molecular pathological features are IDH wild type type, histone H3 wild type.
According to the characteristics of DNA methylation, it can be divided into RTK1, RTK2, and MYCN. RTK2 has high frequency of EGFR amplification.
Homozygous deletion with CDKN2A/B, the prognosis is better; MYCN type with high frequency of MYCN amplification and ID2 amplification, the worst prognosis; RTK1 type with high frequency of PDGFRA amplification.
(3) Infantile hemispheric glioma:
It mainly occurs in infants and young children and is located in the cerebral hemisphere.
Its molecular genetic characteristics are mutations in the receptor tyrosine kinase (RTK) family, mainly including fusion of NTRK family genes (NTRK1/2/3). , ROS1 fusion, MET fusion, ALK fusion.
What is the difference between adult and childhood Diffuse gliomas?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



