The relationship between antibiotics and immunotherapy is reversed!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The relationship between antibiotics and immunotherapy is reversed!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The relationship between antibiotics and immunotherapy is reversed!
With the success of the IMbrave150 trial, the immunotherapy of liver cancer has entered the stage of history [1].
The recently updated 2022 version of the Barcelona staging (BCLC) [2] included atezolizumab in combination with bevacizumab as first-line treatment for patients with advanced HCC.
In clinical practice, patients with liver cancer who use immunotherapy often use a variety of other drugs at the same time.
Several studies have shown that these concomitant drugs may have potential immunomodulatory effects, thereby affecting the efficacy of immune checkpoint inhibitors (ICIs).
In 2018, a well-known medical journal Science published an article in which scholars found that antibiotics interfere with normal intestinal microbial homeostasis, and homeostasis imbalance can lead to primary resistance to immune checkpoint inhibitor (ICI) therapy [3] ].
Subsequent studies by scholars have also found a similar situation. The above situation will lead to the worsening of ICI treatment effect and the increase of immune-related adverse reactions [4, 5].
Current studies on most cancers have shown that the use of antibiotics at the same time as ICI treatment will make the efficacy worse. Will the application of antibiotics also affect the effect of immunotherapy in patients with liver cancer?
To answer this question, Professor David J. Pinato from Hammersmith Hospital, London, UK conducted an international multicenter retrospective study [6].
The study finally found that the application of antibiotics in the early stage of ICI treatment can significantly prolong the progression-free survival time (PFS) of patients , but has no effect on overall survival (OS), objective response rate (ORR) and tumor control rate (DCR).
The findings, published in the journal Liver Cancer , are the first to investigate the effect of antibiotics on immune checkpoint inhibitors in patients with liver cancer.

Figure 1 Title of the article
The study included liver cancer patients from 12 clinical centers around the world from 2017 to 2019. All patients received ICI monotherapy or combination therapy. After treatment, they were evaluated based on RECIST 1.1 criteria [7].
The study divided patients into early antibiotics group (EIOP) and non-early antibiotics group (Non-EIOP). Early antibiotic use was defined as antibiotic use 30 days before or after initial ICI treatment.
As shown in Figure 2, the study ultimately included 449 patients, of which 279 were EIOP and 170 were Non-EIOP. More patients in the Non-EIOP group underwent surgical resection before ICI (Non-EIOP vs EIOP: 71% vs 28%, p=0.0079).
There were no statistically significant differences in other baseline information between the two groups of patients. The main reason for antibiotic use in the EIOP group was fever due to suspected infection.
The most commonly used antibiotics are β-lactam antibiotics and quinolone antibiotics.
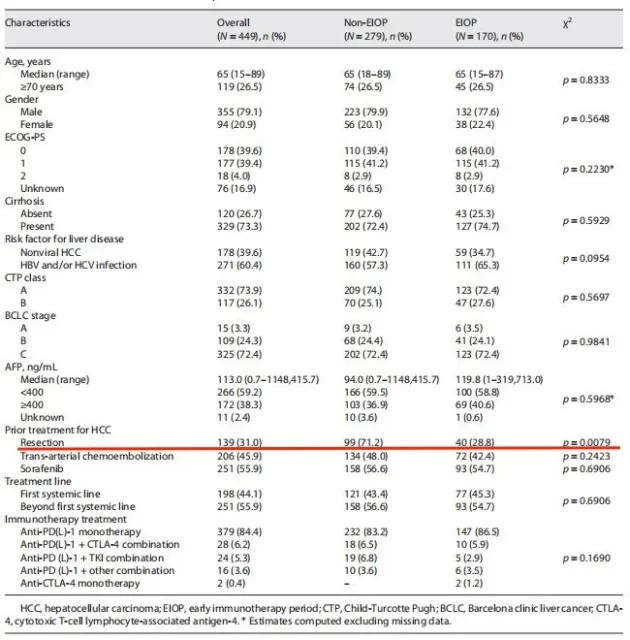
Figure 2 Baseline information table of patients in different groups
The target endpoints of this study include overall survival (OS), progression-free survival (PFS), objective response rate (ORR) and tumor control rate (DCR).
Considering the existence of lead-in time bias (a survival time bias) [8] in this study, which affected the final OS and PFS statistical analysis, the authors used the method of segment survival analysis to correct (Landmark analysis).
Landmark analysis can first identify a specific time point, and then restart the survival analysis from this time point in patients who did not experience an end point event [9].
In this study, the main reason for the lead-in time bias was that some patients in the EIOP group received antibiotics 30 days after receiving ICI treatment, at which time the patients had already applied 30 days of ICI without progression.
At the same time, some patients in the Non-EIOP group may discontinue immunotherapy within 30 days due to ineffective ICI treatment.
When analyzing the efficacy of ICI, the time starting points of the above two types of people will not be on the same starting line, which will inevitably affect the subsequent statistical analysis results.
In this study, the Landmark analysis method uniformly set the time starting point as 4 weeks after receiving ICI treatment. Subsequent analysis of patients who have not progressed at this time will eliminate the above-mentioned survival time bias.
Finally, the authors found that, as shown in Figure 3, both for the overall population and the population after landmark analysis, the EIOP group had a certain benefit in PFS relative to the Non-EIOP group (median PFS: EIOP vs Non-EIOP: 6.1 vs. 3.7, p=0.0135) , while no significant benefit was seen in OS (median OS: EIOP vs Non-EIOP: 15.3 vs 15.4, p=0.6275).
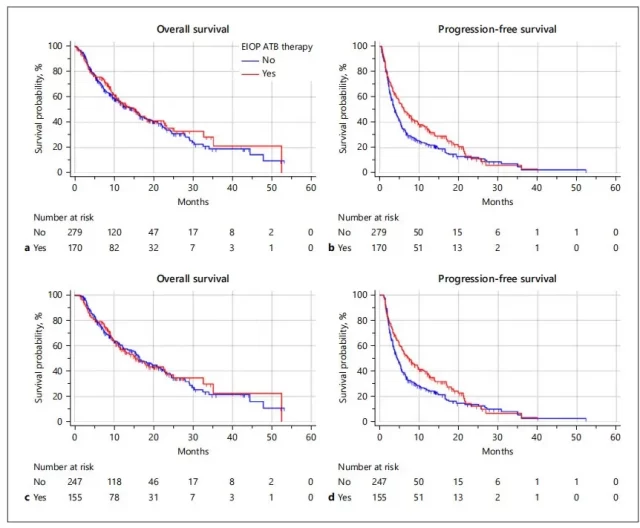
Figure 3 Effects of different EIOP groups on OS and PFS of patients
Considering that this study is a retrospective study, the differences in baseline information between patients in the EIOP group and the Non-EIOP group may affect the final statistical results. The authors applied multivariate regression analysis to adjust for confounding factors.
The specific variables included in the analysis are shown in Figure 4. As can be seen from Figure 4, after adjusting for other confounding factors, patients in the EIOP group still benefited from PFS , while the effects of OS, ORR, and DCR were not statistically significant.
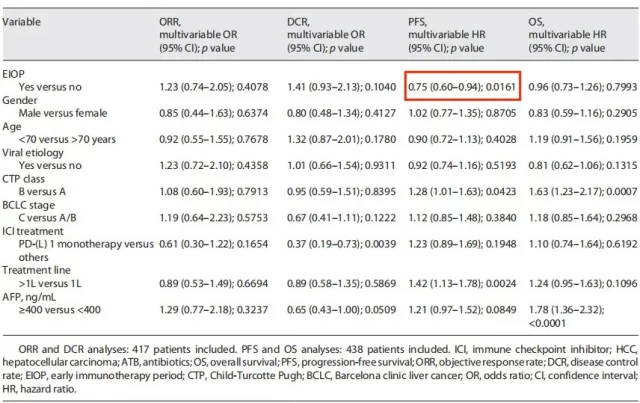
Figure 4 Multivariate regression analysis results after correction
Finally, to further demonstrate the robustness of the results, the authors performed a subgroup analysis, and they limited the study population to those receiving ICI monotherapy and those with liver function CTP class A, respectively .
As can be seen in Figure 5, in both subgroup analyses, EIOP benefited patients in terms of PFS.
In the subgroup of patients receiving ICI monotherapy, patients in the EIOP group had a higher disease control rate (DCR: EIOP vs Non-EIOP: 61.4 vs 50.9, p=0.0494)
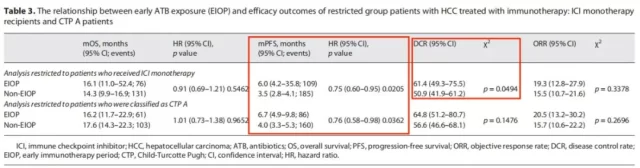
Figure 5 Subgroup analysis results
This study found that for patients with liver cancer, the application of antibiotics in the early stage of immunotherapy could prolong the progression-free survival of patients, but had no significant effect on overall survival, disease control rate and objective response rate.
In patients with other malignant tumors treated with ICI, the application of antibiotics generally reduces the efficacy of ICI on tumors or increases the toxic and side effects of ICI therapy [10]. However, the present study found that the opposite is true in liver cancer. The authors believe that liver cancer occurs on the basis of liver cirrhosis, and the cirrhotic state has been associated with the immunosuppressive state of the liver caused by gut microbial disturbances [11]. Studies have shown that cirrhosis is associated with a decrease in beneficial bacteria and an increase in immunosuppressive bacteria in the gut. Therefore, the authors hypothesized that administration of antibiotics might interfere with this immunosuppressive state caused by an increase in immunosuppressive bacteria to enhance the efficacy of ICI. Further prospective studies and related gut microbiota studies are needed in the future to further explore the reliability and underlying mechanism of the conclusions.
references:
1. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020. 382(20): 1894-1905.
2. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation Barcelona Clinic Liver Cancer (BCLC) staging system. The 2022 update. J Hepatol. 2021 .
3. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018. 359(6371): 91-97.
4. Iglesias-Santamaría A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin Transl Oncol, 2020,22(9):1481-1490.
5. Huang XZ, Gao P, Song YX, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology, 2019,8(12):e1665973.
6. Fessas P, Naeem M, Pinter M, et al. Early Antibiotic Exposure Is Not Detrimental to Therapeutic Effect from Immunotherapy in Hepatocellular Carcinoma. Liver Cancer, 2021,10(6):583-592.
7. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1). Eur J Cancer, 2009, 45(2):228-47.
8. Peng Xiaoxia, Shu Xiaochen, Tan Jing, Wang Li, Nie Xiaolu, Wang Wen, Wen Zehuai, Sun Xin. Technical Specifications for Observational Study Design for Evaluation of Treatment Outcomes Based on Real-World Data. China Journal of Evidence-Based Medicine. 2019; 19:779-86 .
9. Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. 2018;31:125-30. https://doi.org/10.1111/tri. 13081
10. Spakowicz D, Hoyd R, Muniak M, Husain M, Bassett JS, Wang L, Tinoco G, Patel SH, Burkart J, Miah A, Li M, Johns A, Grogan M, Carbone DP, Verschraegen CF, Kendra KL, Otterson GA, Li L, Presley CJ, Owen DH. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: causal modeling, timing, and classes of concomitant medications. BMC Cancer. 2020;20:383. https: https://doi.org/10.1186/s12885-020-06882-6
11. Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, Segata N, Desnoyer A, Pietrantonio F, Ferrere G, Fahrner JE, Le Chatellier E, Pons N, Galleron N, Roume H, Duong C , Mondragón L, Iribarren K, Bonvalet M, Terrisse S, Rauber C, Goubet AG, Daillère R, Lemaitre F, Reni A, Casu B, Alou MT, Alves Costa Silva C, Raoult D, Fizazi K, Escudier B, Kroemer G , Albiges L, Zitvogel L. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur Urol. 2020;78:195-206. https://doi.org/10.1016/j.eururo.2020.04.044
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



