The causal relationship: T cell differentiation and anti-tumor efficacy
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The causal relationship: T cell differentiation and anti-tumor efficacy
The causal relationship: T cell differentiation and anti-tumor efficacy. For many years, which T cell subsets should be the target of ACT has been the focus of debate and debate. Because the ultimate goal of adoptive immunotherapy is to produce T cells that can spread all over the body.
In the search for metastatic cancer deposits for destruction, it was initially assumed that the most ideal T cells should have the tendency to naturally infiltrate the surrounding tissues and have direct cell lysis capabilities.
In fact, this will mean that the CD62L TEM and TEFF populations will be the preferred types of cell transfer. However, this hypothesis is inconsistent with empirical clinical data, which suggests that the transfer of less differentiated T cells is associated with tumor response.
Therefore, a systematic study was carried out in preclinical animal models to determine whether there is a causal relationship between the differentiation state of T cells and the anti-tumor efficacy at the time of infusion (Figure 1).
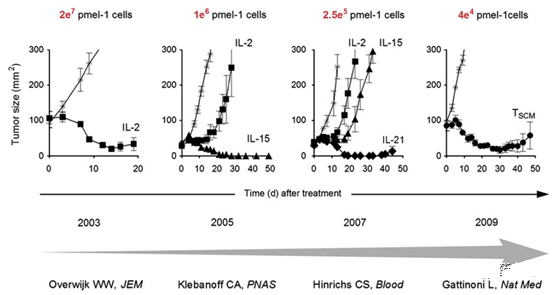
Figure 1. Iterative progress in using pmel-1 mouse model to enhance the efficacy of adoptive CD8+ T cell therapy in the treatment of melanoma; the use of alternative γc cytokines or less differentiated T cell subsets has resulted in tumor-reactive CD8+ T cells The therapeutic efficacy of the drug is gradually improved, thereby providing effective tumor destruction ability by transferring a limited number of cells.
In a set of experiments, murine CD8+ T cells from pmel-1 T cell receptor (TCR) transgenic mice were stimulated in vitro to generate cells that occupy late stages of differentiation, called early, intermediate, or late effectors. Consistent with its higher maturity state, the middle and late stages of TEFF have strong IFNγ release and cytolytic abilities, and up-regulate the expression of key transcription factors (TFs) related to effector differentiation, including Eomes (46) and Id2 and replicative senescence Mark killer cell lectin-like receptor G1 (KLRG-1).
On the contrary, as the degree of differentiation of T cells increased, they lost the ability to release IL-2 and down-regulated the expression of a variety of cell surface markers, including CD62L, CCR7, CD27 and IL-7Ra (CD127). In addition, the re-stimulated T cells reduced the expression of naive-related TFs, such as Id3. When the middle and late stages of TEFF are transferred in vivo, these cells have poor expansion and durability, and most importantly, their ability is greatly impaired, leading to tumor regression in the early stage relative to TN and TEFF.
At the same time, experiments were conducted to evaluate the ability of conventional CD8+ T cell memory subpopulations to mediate cancer regression after ACT (Figure 2). Initially, the anti-tumor efficacy of tumor-responsive TCM versus TEM was compared (24). Similar to the results observed in the virus challenge model, TCM has higher in vivo proliferation and survival ability than TEM after inoculation with homologous tumor Ag. Although both T cell memory subsets can mediate cancer regression after ACT, only TCM induced a complete response at the tested cell dose, and the mice that received TEM eventually died of uncontrolled tumor growth.
Subsequently, when the limited cell dose was about 2 orders of magnitude less than in previous experiments, the ability of TSCM to mediated cancer regression was directly compared with that of TCM and TEM populations. Consistent with earlier results, TCM mediates excellent in vivo expansion, durability and anti-tumor efficacy. However, in terms of each unit, TSCM is more powerful than TCM.
When evaluating tumor regression ability as a function of the number of input T cells, there is a significant linear correlation between T cell differentiation. The status and anti-tumor efficacy are found in the order of TSCM>TCM>TEM. These results are confirmed in a separate, vaccine-independent tumor treatment model system, in which a subpopulation of human T cells genetically engineered expresses the anti-mesothelin chimeric antigen receptor, in immunodeficient mice Treatment of human mesothelioma xenotransplantation.
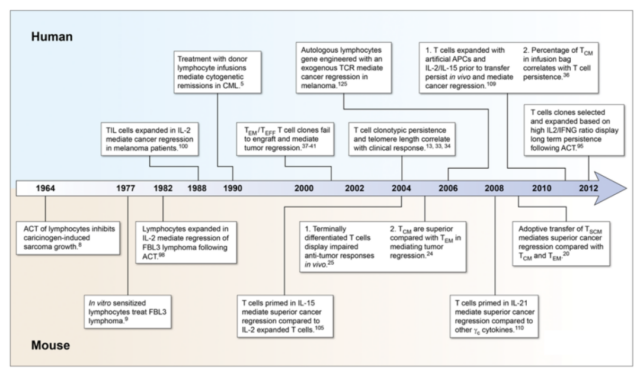
Figure 2. A timeline for understanding the progression of T cell quality associated with effective adoptive immunotherapy to treat cancers in mice and humans.
The ability of naturally occurring Ag-specific genetically engineered TEFFs derived from different CD8+ T cell subpopulations was evaluated. Initially, the relative implantation efficiency of TEFF obtained from a conventional memory subset was studied (Figure 2). In immunodeficiency mouse models and non-human primates that received human T cell transfer, TEFF derived from TCM precursors showed higher durability than TEM-derived TEFF.
It is worth noting that despite the following facts, these differences are still observed: Both memory-derived TEFF subpopulations have a highly differentiated phenotype upon cell transfer, which is characterized by low expression of CD62L, CCR7, CD28 and CD127 And the high expression of granzyme B and perforin.
These data indicate that the current cell surface marker set used to characterize T cell subpopulations lacks important heterogeneity, which may be due to the genetics of T cells that cannot be distinguished from other phenotypes on a single epidermal cell. Genetics, or metabolic characteristics at the cellular level.
Although TCM generally only represents a small number of people in humans, TN is generally the main population in the peripheral circulation. In addition, TN has longer telomeres and therefore has greater replication capacity compared to the subpopulations with Ag experience. For these reasons, the phenotype, function and anti-tumor ability of TEFF derived from the TN but not the TCM population have been evaluated in mice and humans. Compared with the results of comparing TEFF effector cells derived from memory, TN is still different from TCM-derived TEFF in phenotype and function.
The naive TEFF retains the ability to release IL-2 while preventing the acquisition of the aging marker KLRG-1. In contrast, TEFF derived from TCM lost the ability to secrete IL-2 and significantly up-regulated the expression of KLRG-1. In addition, compared with TEFF from TCM sources and TEM sources, human-born CD8+ TEFF exhibits superior retroviral transduction efficiency for exogenous TCR, and the telomere length is significantly extended. When transferred to tumor-bearing mice, TEFF compared to TCM-derived TEFF, monoclonal antibodies derived from naive cells have better in vivo expansion, durability and anti-tumor efficacy in vivo.
Overall, these data confirm that TEM represents an inferior T cell population of adoptive immunotherapy, and that a part of RA+ in the CD62L+ subpopulation should be retained instead of focusing on the isolation, expansion, and reinjection of cells derived only from the TCM subpopulation .
Finally, it should be pointed out that the conclusion that less differentiated T cells is better than more differentiated T cells in mediating anti-tumor immunity is not limited to CD8+ T cells, but also seems to be extended to CD4+ T cells. Gene expression profiles of memory CD8+ T cells that were repeatedly stimulated in vivo based on heterologous vaccine constructs were used for gene set enrichment analysis.
Gene expression profiles of memory CD8+ T cells that were repeatedly stimulated in vivo based on heterologous vaccine constructs were used for gene set enrichment analysis, and the gene expression profiles of Th17 cells were highly enriched with genes related to primary CD8+ memory T cells. On the contrary, the expression profile of Th1 cells enriched the late-stage memory CD8+ T cells obtained after multiple rounds of in vivo stimulation. In multiple model systems, adoptively transferred Th17 cells have proliferative, durable and mediated anti-tumor immunity advantages over Th1 cells.
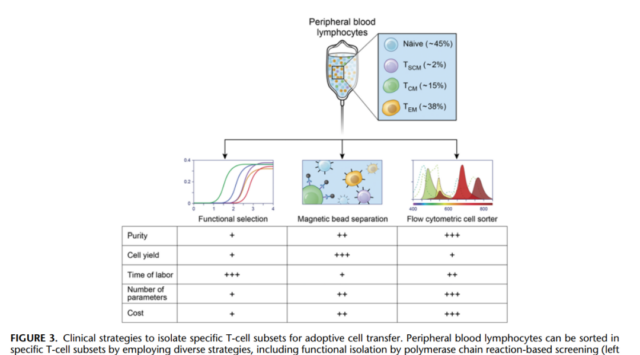
In summary, the results found in mice, non-human primates and humans have established a causal inverse relationship between T-cells, cell differentiation status and metastatic T cell engraftment, proliferation, and relative ability to mediate anti-tumor immunity . These data strongly support the use of the less differentiated CD62L+TN, TSCM and TCM subgroups on CD62L TEM and TEFF for adoptive immunotherapy.
It is found that the gene expression profile of Th17 cells is highly enriched in genes related to primary CD8+ memory T cells. On the contrary, the expression profile of Th1 cells enriched the late-stage memory CD8+ T cells obtained after multiple rounds of in vivo stimulation. In multiple model systems, adoptively transferred Th17 cells have proliferative, durable and mediated anti-tumor immunity advantages over Th1 cells.
The results found in mice, non-human primates and humans have established a causal inverse relationship between T-cells, cell differentiation status and metastatic T cell engraftment, proliferation, and relative ability to mediate anti-tumor immunity . These data strongly support the use of the less differentiated CD62L+TN, TSCM and TCM subgroups on CD62L TEM and TEFF for adoptive immunotherapy.
The causal relationship: T cell differentiation and anti-tumor efficacy
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



