Chronic immunotoxicity of immune checkpoint inhibitors
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Chronic immunotoxicity of immune checkpoint inhibitors
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Chronic immunotoxicity of immune checkpoint inhibitors.
Immune checkpoint inhibitors ( ICIs ) have become a core pillar of cancer treatment, with almost half of patients with metastatic cancer eligible for ICIs in economically developed countries.
As of December 2021, there are eight approved drugs for 17 different malignancies, and these drugs are increasingly used in multiple ( neo )adjuvant and maintenance treatments.
ICIs are also frequently used in combination regimens, including those involving other classes of ICIs, chemotherapy, cell therapy, and/or targeted therapy.
As such, durable responses are becoming more common, and it is therefore increasingly important to describe the long-term physiological implications of ICIs therapy.
Patients with durable responses appeared to have broadened peripheral T- and B-cell repertoires and appeared to develop immune memory compared to patients who relapsed after treatment.
The molecular basis of these responses is not fully understood, but regardless of their mechanism of action, the prolonged duration of the therapeutic effect of ICIs often far exceeds their pharmacokinetic half-life.
This long-lasting pharmacodynamic effect also has implications for toxicity.
Emerging evidence suggests that persistent toxicity may be more common than expected, and although these chronic sequelae are usually low-grade, they affect endocrine, rheumatic, pulmonary, neurological, and other organ systems.
Fatal toxicity also includes a variety of clinical manifestations and can occur in 0.4–1.2% of patients. This risk is a particularly important consideration given the likelihood of a patient’s long-term survival.
In addition, immune checkpoint blockade has effects on multiple immune processes, including atherosclerosis, heart failure, neuroinflammation, obesity, and hypertension, which have not been described but remain an important area of research that may be associated with cancer survival related to.
Immune activation and irAEs
Immune activation of the majority of immune-related adverse effects ( i rAEs ) may be associated with activity required for antitumor immune responses, a tumor-specific hypothesis supported by a reproducible positive relationship between treatment response and incidence of irAEs. However, there is also evidence that irAEs have mechanisms unrelated to antitumor activity, including those involving microbiome, virus, or tissue-specific factors.
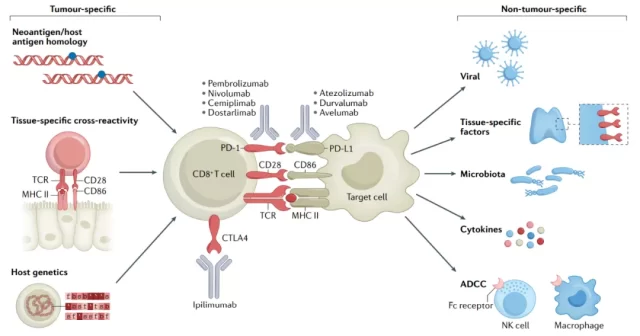
Different ICI regimens have different toxicity profiles, with high-grade irAEs typically occurring in a dose-dependent manner for regimens containing anti-CTLA4 antibodies ( 30–55% for the combination of ipilimumab plus nivolumab ), but not for anti-CTLA4 antibodies used as monotherapy.
PD-1–PD-L1 antibodies ( 10–15% incidence ), not dose-dependent.
irAEs can occur in essentially any organ system, including the heart, bone marrow, kidneys, bones, pituitary and other organs. irAEs occurred most frequently during the first three months of treatment, but could occur at any time during treatment, even a few months after the end of treatment.
Despite the lack of strong evidence from randomized clinical trials, acute severe IRAE can be managed reasonably and effectively by providing symptom management, discontinuation of ICIs, and administration of high-dose corticosteroids.
Most irAEs occurred early in the treatment course, but delayed events, i.e., irAEs that occurred after 1 year of treatment, were also possible.
In a study involving 118 patients, the estimated incidence of high-grade, delayed irAEs was 5.3%. The most common late-onset irAEs were colitis, rash, and pneumonitis, and two patients died.
Notably, the majority of patients ( 74% ) received anti-PD-1 antibody therapy at the onset of irAEs, 12% discontinued therapy within the first 3 months, and 14% discontinued therapy beyond 3 months.
These observations reinforce the idea that irAEs ( acute or delayed ) may occasionally occur after discontinuation, but typically occur during active treatment.
Chronic irAEs
Acute irAEs have received most of the attention so far due to their more clinically significant presentation requiring urgent treatment.
However, the data suggest that chronic irAEs are more prevalent than previously recognized.
There is a lack of general consensus on the definition of chronic irAEs, which are defined here as irAEs lasting ≥12 weeks after cessation of treatment with ICIs.
According to this definition, approximately 43% of patients had at least one chronic irAE.
There may be several reasons for the lack of consensus on chronic irAEs. First, most acute irAEs improve at least with steroids and usually resolve with alternation therapy.
Second, adverse event reporting in clinical trials tends to focus on the most common treatment-related toxicities, so low-frequency events are often underestimated or ignored.
Third, most preliminary clinical trials have recruited patients with metastatic cancer, and characterizing chronic and long-term events in patients with metastatic disease is challenging because these patients often have limited life expectancy, thus limiting long-term follow-up.
Such patients may also receive subsequent systemic therapy, surgery, and/or radiation, making attribution of toxicity more difficult.
Finally, the presence of multiple comorbidities common in cancer patients may further impair the identification of chronic irAEs.
Despite these challenges, however, the durable clinical responses observed in a subset of patients treated with ICIs have enabled some follow-up studies to yield preliminary insights.
In these studies, endocrine disorders ( eg, hypothyroidism and emerging type 1 diabetes ) and rheumatic viral diseases ( eg, arthritis ) emerged as the most common chronic irAEs.
Various other low-incidence events have also occurred, including neurological disorders, dermatitis, and pneumonia.
Endocrine irAEs
The earliest identified chronic irAEs are those affecting the endocrine organs and occur in 15–40% of patients treated with ICIs.
Hypothyroidism is the most common endocrine irAE, occurring in approximately 10% of patients receiving anti-PD-1/PD-L1 as monotherapy and up to 20% of patients receiving ipilimumab plus nivolumab.
Hypophysitis is almost unique to patients treated with ICIs. This occurred more frequently ( 5–10% ) and earlier ( median 9–12 weeks ) in patients receiving ipilimumab than in patients receiving anti-PD-1/PD-L1 antibodies. 72.
The preference of patients receiving ipilimumab appears to be related to the expression of CTLA-4 on pituitary hormone-secreting cells, leading to antibody and complement fixation.
Other endocrine irAEs, although less common, are similarly persistent. ICI-induced diabetes mellitus ( ICI–DM ) occurs in <1% of treated patients but may manifest as diabetic ketoacidosis, often requiring lifelong insulin supplementation.
In stark contrast to hypophysitis, ICI-DM is seen almost exclusively in patients receiving anti-PD-1/PD-L1 antibodies and rarely in patients receiving anti-CTLA-4 monotherapy.
RheumatismirAE
The emergence of various rheumatic irAEs does not seem surprising, given that rheumatism falls within the spectrum of autoimmune diseases.
Interestingly, systemic lupus erythematosus and mixed connective tissue disease have so far not been associated with ICIs, as can occur in RA, polymyalgia rheumatica, polymyositis, and Sjögren syndrome.
About half of patients experience persistent arthritis symptoms lasting at least 6-12 months after discontinuation of ICIs.
Data from a small range suggest that ICI-induced chronic arthritis has a large negative impact on quality of life, at least as much as other irAEs.
Among other rheumatic irAEs, ICI-induced Sjögren’s syndrome has both similarities and differences with Sjögren’s syndrome in that xerostomia predominates rather than ocular involvement.
A major challenging issue in rheumatic irAEs is the management of chronic low-grade toxicity, which applies to multiple organ systems but is most pronounced in the context of rheumatic irAEs.
Rheumatic irAEs may impair quality of life, but are usually not sufficient to sustain high-dose steroids or discontinue ICIs.
Therefore, in the setting of ICI therapy, patients usually continue to receive low-dose steroids and/or antirheumatic drugs ( DMARDs ).
Gastrointestinal irAEs
In patients treated with anti-PD-1 antibodies, colitis occurs in up to 5% of patients and usually presents with diarrhea and less often abdominal pain and blood in the stool.
However, diarrhea of any grade ( 44% ) or severe diarrhea/colitis ( 15% ) occurred more frequently in patients receiving the ipilimumab-containing regimen.
Hepatitis occurs in 3-10% of patients, is asymptomatic or may present with nonspecific symptoms, including malaise and myalgia, and less often jaundice and acute liver failure.
Delayed diarrhea may also reflect pancreatic insufficiency.
In one cohort, a 1% incidence of steatorrhea was reported in patients receiving anti-PD-1 antibodies, with a median time to onset of 9 months.
Notably, 10% of this group of patients had imaging signs of pancreatic atrophy, although the mechanism and clinical significance of this finding in asymptomatic patients remains to be seen.
Pulmonary irAEs
Pneumonia is one of the major sources of anti-PD-1 antibody morbidity and mortality, most commonly manifested as dry cough, hypoxia, and bilateral ground-glass opacities ( including interstitial and organizing pneumonia ).
Although most patients have persistent imaging findings for at least 1-2 years after symptom onset, most of these patients have resolved their symptoms.
In addition, reactivation of tuberculosis may also occur in rare patients with chronic wheezing or coughing.
Data from retrospective studies suggest similar rates in cancer patients who did not receive ICIs, so it is unclear whether ICIs play a role in this effect.
Cardiovascular irAEs
Acute fulminant myocarditis, the earliest known cardiovascular irAE associated with ICI, typically presents with ECG disturbances, including arrhythmias and concurrent myositis.
Other recognized cardiac sequelae include pericarditis, which is more common in lung cancer patients receiving anti-PD-1/PD-L1 antibodies, is usually less fulminant, and is more sensitive to corticosteroids.
Vascular irAEs have also been described, including acute vasculitis, especially temporal arteritis, and polymyalgia rheumatica.
Collectively, these events may occur in as many as 1–2% of treated patients.
Combination regimens involving ICIs and other conventional or targeted therapies are another important area of research, both of which may have their inherent chronic cardiotoxicity.
Similarly, in patients with RCC and several other cancers, ICIs are often used in combination with vascular endothelial growth factor inhibitors such as axitinib or lenvatinib , which are associated with a number of cardiovascular sequelae, including hypertension, Vascular disease and cardiomyopathy.
Neurogenic irAEs
Neurogenic irAEs occur in up to 5% of patients and are more common with ipilimumab-containing regimens.
These events can affect the neuromuscular junction ( myasthenia gravis and Lambert–Eaton myasthenic syndrome ), the central nervous system ( meningoencephalitis ), and peripheral nerves ( sensory and motor neuropathies, including Guillain-Barré syndrome ).
Meningoencephalitis usually resolves with acute treatment, and it is unclear whether some patients develop chronic deficits.
The long-term prognosis of ICI-related myasthenia gravis is unclear, although in the majority of patients, ICI-related myasthenia gravis appears to be either in complete remission or controlled with disease-specific therapy.
ICI-related Guillain-Barré syndrome has a high case fatality rate ( 19% ), and even patients who do improve symptoms through immunomodulation often have residual weakness and/or sensory loss.
Peripheral neuropathy appears to be the neurological disorder most likely to develop chronic symptoms.
Chronic peripheral neuropathy was reported in approximately 2% of patients who received adjuvant anti-PD-1 antibody therapy for resected melanoma.
skinirAE
Skin toxicity is one of the most common complications in patients with ICIs, including various inflammatory dermatitis syndromes, pruritus, and vitiligo.
These events often present a challenging clinical dilemma because they are disturbing but not life-threatening, and there appear to be insufficient symptoms to stop treatment or require high doses of steroids.
Patients with ICI-induced dermatitis or pruritus may require continued use of antihistamines, topical steroids, and/or GABAergic agonists.
Based on experience, these IRAEs tend to resolve eventually, although dermatitis and pruritus typically persist for weeks or months after discontinuation of the drug.
Vitiligo is more common in people with melanoma than other cancers, is often incurable, and can be a lifelong complication.
Severe skin reactions, such as Stephens-Johnson syndrome and bullous pemphigoid, can also have life-threatening long-term consequences.
Other irAEs
Similar to acute IRAEs, a broad series of low-frequency events may eventually become chronic irAEs.
For example, nephritis, in one series, about 10% of patients required hemodialysis, and half of these patients failed to restore adequate renal function and required renal replacement therapy.
Hematologic toxicities, including idiopathic thrombocytopenic purpura, aplastic anemia, hemophagocytic lymphangiohistiocytosis, and pure red cell aplastic anemia, may also occur, although infrequently, may be fatal .
Ocular symptoms may include uveitis and conjunctivitis.
Other symptoms, such as fatigue, may also persist after the ICI is discontinued.
The pathophysiology, incidence, and time course of these nonclassical irAE sequelae have not been systematically studied.
lethal irAEs
Fatal irAEs are rare and may result from severe autoinflammation that is refractory to steroids and/or other immunosuppressive agents.
Although these events clearly fall into a different category from chronic toxicity, the risk of fatal events requires special consideration in the context of the potentially persistent responses produced by ICIs.
The data showed a lower incidence of fatal irAEs, ranging from 0.4% with anti-PD-1/PD-L1 antibody monotherapy to about 1.2% with anti-CTLA-4/PD-1 combination regimens.
Despite some incidence, these mortality rates are relatively low compared to mortality rates associated with cytotoxic chemotherapy, molecularly targeted therapy, and even high-risk surgery for cancer.
Death may be caused by various organ-specific irAEs. Myocarditis is the irAE with the highest fatality rate ( 25–50% of patients ), mainly due to refractory arrhythmias in critically ill patients.
Myositis can also be accompanied by myocarditis, resulting in paralysis of the diaphragm and respiratory failure. Pneumonia is fatal in 10-15% of patients, usually due to respiratory failure.
Hepatitis ( caused by fulminant liver failure ) and colitis ( caused by perforation of the colon ) can also lead to death, although the incidence is usually much lower.
Neurogenic irAEs, although uncommon, can also be fatal in 10-15% of patients, usually due to refractory or prolonged Guillain-Barre syndrome, encephalitis, or myasthenia gravis-like syndrome.
Notably, fatal irAEs typically occurred early in the treatment course, with a median of 15 days ( combination therapy ) and 40 days ( anti-PD-1 antibody as monotherapy ), suggesting a pre-existence of rapid release after ICI initiation Organ-specific inflammation may be responsible for these events.
In addition, a trend toward older age in patients with fatal irAEs was also observed, suggesting that older age and associated reduced functional reserve may predispose patients to death.
Long-term immunosuppression may be a direct cause of death in patients with chronic refractory toxicity, as the presence of opportunistic infections may also complicate the clinical course.
Reactivation safety
Another long-term effect of irAEs is whether patients who experience some benefit from treatment but also have severe toxicity should be challenged again after the irAE has resolved.
Clinicians may seek to re-challenge a subset of patients, including those with early-onset toxicity who may benefit from further treatment, or those who remain responsive when ICIs are discontinued and later develop disease progression.
Prospective data on the safety of rechallenge are currently lacking.
Data from retrospective studies suggest that IRAE recurs in approximately 25–50% of patients who are re-treated with anti-PD-1/PD-L1 antibodies.
Determining which patients are at the highest risk for recurrent IRAEs is currently challenging.
Data from one series showed that after rechallenge, colitis, pneumonitis, and hepatitis had higher recurrence rates than other irAEs, and older age was also associated with irAE recurrence.
A longer delay between discontinuation and rechallenge may reduce the risk of recurrent toxicity ( eg, in patients re-treated after discontinuation for irAEs for more than 12 months ).
Clinicians should consider the type and severity of irAEs when making rechallenge decisions.
A theoretical approach that has not yet undergone extensive clinical trials is to restore ICIs in combination with selective immunomodulatory therapy.
For example, targeted inhibition of cytokines such as IL-6 could theoretically decouple antitumor immune responses from irAEs and allow safe re-challenge.
Several clinical trials are currently testing these approaches ( NCT03293784, NCT03999749 and NCT04940299 ).
Summary
ICIs have durable responses and often manageable toxicity in a variety of cancers, making them an attractive and widely used treatment option for cancer patients.
This broad application broadens treatment-related and survival considerations beyond just generating an antitumor immune response, leading to lifelong impacts on quality of life.
Therefore, in addition to typical irAEs, it is very important to understand chronic irAEs that block immune checkpoint molecules and their long-term effects on overall immune function.
References :
1.Immune-checkpoint inhibitors: long-termimplications of toxicity. Nat Rev Clin Oncol. 2022 Jan 26 : 1–14.
Chronic immunotoxicity of immune checkpoint inhibitors
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



