Solving the Mystery of Radiotherapy Resistance of Brain Metastasis Cancer Cells
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
Solving the Mystery of Radiotherapy Resistance of Brain Metastasis Cancer Cells
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Nature Medicine: Solving the Mystery of Radiotherapy Resistance of Brain Metastasis Cancer Cells
Whole brain radiation therapy (WBRT) has been one of the main treatments for brain metastases, especially for those patients who do not have the opportunity for surgery [1]. Unfortunately, current clinical evidence shows that whole brain radiotherapy does not significantly improve the prognosis of patients with brain metastases [2].
Therefore, exploring the molecular mechanism of radiotherapy resistance in brain metastases and finding molecular markers that can predict radiotherapy sensitivity will help to find solutions to radiotherapy resistance and formulate individualized radiotherapy regimens for patients.
Recently, the team of Professor Manuel Valiente from the Spanish National Cancer Research Center discovered a new mechanism of resistance of brain metastases to whole-brain radiotherapy.
They found that brain metastases cancer cells can highly express and secrete S100A9 protein, which mediates radioresistance by binding to the receptor RAGE on the surface of tumor cells and activating the intracellular NF-κB/JunB signaling pathway. reversed by the RAGE inhibitor FPS-ZM1 .
In addition, they also found that S100A9 can be used as a biomarker (both blood test or tumor tissue test) to predict the sensitivity of patients with brain metastases to whole-brain radiotherapy, so as to personalize radiation therapy for patients .
The results of this study provide a theoretical basis for individualized radiation therapy for patients with brain metastases. At the same time, a radiosensitizer with potential clinical application value was discovered, which has important clinical translation significance. The relevant research results were published in the famous journal Nature · Medicine” on [3].
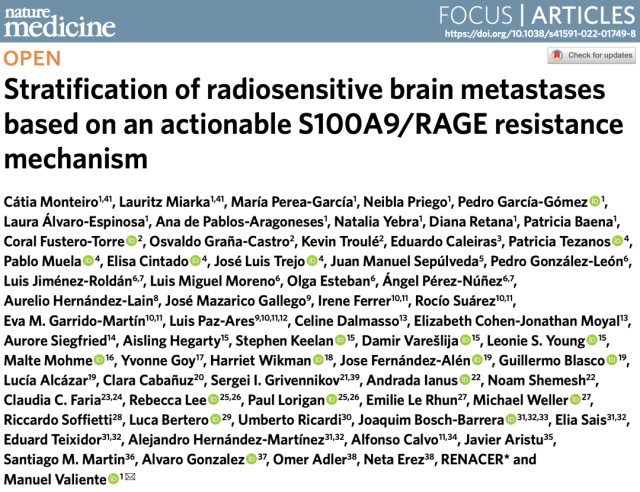
In order to study the mechanism of brain metastases (BrM) resistance to whole-brain radiotherapy, Professor Manuel Valiente’s team first constructed an animal model of brain metastases from lung cancer (intracardiac inoculation of lung adenocarcinoma cell line H2030-BRM) and an animal model of brain metastases from breast cancer (TNBC ). cell line E0771-BRM inoculated intracranially) .
They found that three different regimens of whole-brain radiation did not prolong survival in mice compared with controls , and that both cell lines were surprisingly sensitive to radiation when cultured in vitro .
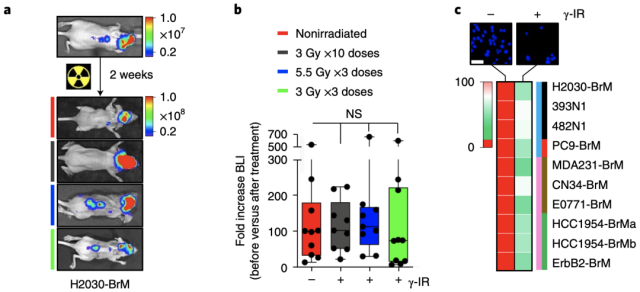
An animal model of brain metastases is resistant to whole-brain radiotherapy, but cell lines are sensitive to radiotherapy when cultured in vitro
This intriguing phenomenon has led researchers to wonder if it is because tumor cells come into contact with brain tissue that they become resistant to radiotherapy?
So they placed H2030-BRM or E0771-BRM cells on thin slices of brain tissue grown in vitro and treated them with the same radiation as before, and found that the cancer cells did become resistant to radiation, suggesting that the brain where the tumors grew Substances that alter the sensitivity of cells to radiotherapy exist in the external microenvironment .
Previous studies have found that the interaction between brain metastatic cancer cells and glial cells plays an important role in the growth of brain metastatic cancer cells [4]. After co-culturing brain metastatic cancer cells and glial cells, the researchers found that only when the brain metastases cancer cells were in direct contact with glial cells (separate culture of the two cells was ineffective) , especially with astrocytes, the Resistance to radiotherapy occurs .
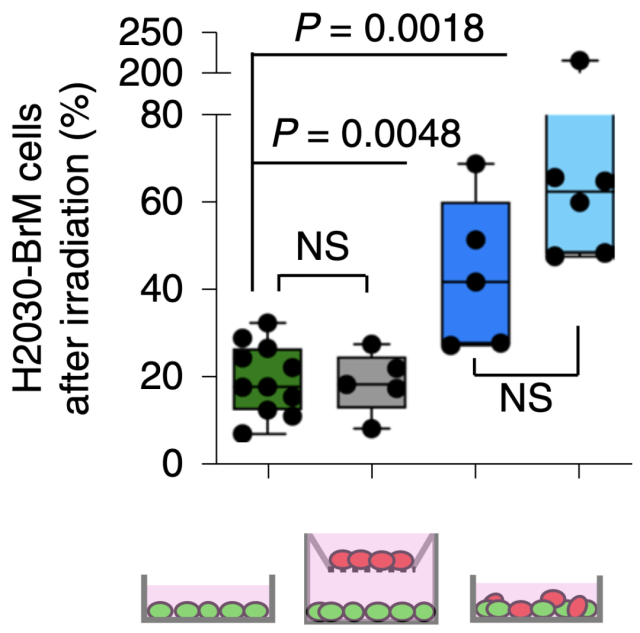
Brain metastases cancer cells come into contact with astrocytes to develop resistance to radiotherapy
To study the molecular mechanism, the researchers performed RNA sequencing on control cells and co-cultured brain metastatic cancer cells, and found that S100A9 was the most significantly up-regulated gene, and S100A9 was also highly expressed in animal models and human brain metastatic cancer specimens .
The high expression of S100A9 is induced by CXCL1 and TGG-α secreted by astrocytes. Adding the above two cytokines to the cells cultured in vitro can induce the high expression of S100A9 in brain metastatic cancer cells and become resistant to Radiotherapy resistance.
The above studies suggest that S100A9 may be involved in the mechanism of radiation resistance of brain metastatic cancer cells .
To test this, the researchers added recombinant S100A9 to radiosensitive brain metastases cancer cell lines in vitro and found that tumor cells were 3-fold more sensitive to radiotherapy. However, after silencing the cells S100A9 and then culturing them with brain tissue slices, the tumor cells were still sensitive to radiotherapy. This confirms that S100A9 is the “culprit” that causes tumor radiotherapy resistance .
So how does S100A9 cause radiosensitized brain metastases to become radioresistant?
Through transcriptomic analysis, the researchers found that the receptor RAGE of S100A9 was highly expressed after radiation stimulation in radiotherapy-resistant brain metastases, and also in animal models and human brain metastases, with enriched pathways. Analysis showed that the NF-κB signaling pathway downstream of RAGE was significantly activated.
These data suggest that brain metastases cancer cells express and secrete S100A9, which binds to the radiation-induced high expression of the RAGE receptor and activates NF-κB to mediate radiation resistance .
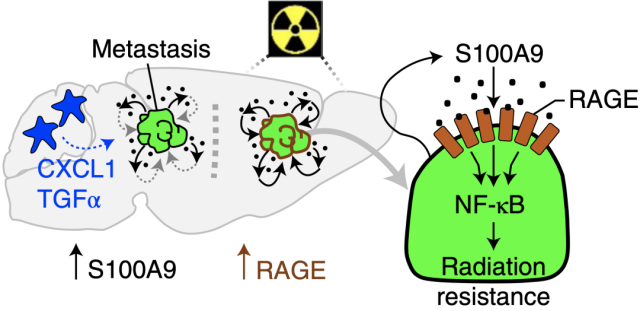
Brain metastases cancer cells mediate radioresistance through S100A9
Next, the researchers studied the effect of S100A9 on radiosensitivity in vivo, and constructed a lung cancer brain metastasis model in which H2030-BrM cells were silenced by S100A9.
The results showed that tumor resistance to radiotherapy disappeared , and radiotherapy significantly prolonged the survival of mice compared with the control group (H2030-BrM cell wild-type lung cancer brain metastasis model) . A similar phenomenon was observed in animal models of breast cancer brain metastases.
The researchers then confirmed in vivo that S100A9 indeed mediates tumor radioresistance by activating the NF-κB/JunB signaling pathway .
Since S100A9 can be expressed not only by tumor cells, but also secreted by neutrophils and monocytes. To verify whether radioresistance is mediated by tumor-specifically secreted S100A9, the researchers constructed a S100A9 knockout mouse model, and then implanted wild-type E0771-BRM cells into the brain to construct a brain metastasis model. Radiotherapy remained resistant. Thus , it was confirmed that radioresistance was indeed mediated by S100A secreted by brain metastatic cancer cells .
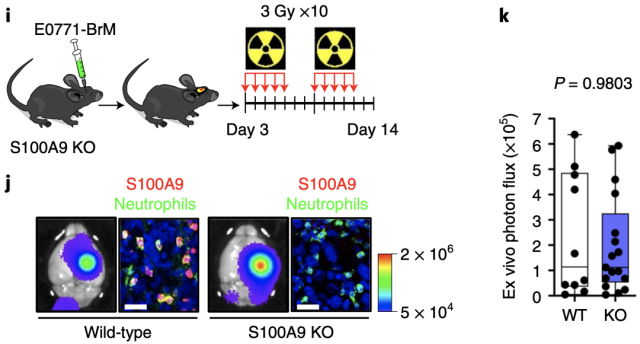
S100A9 knockout mouse model of brain metastases is resistant to radiotherapy
Given the association of S100A9 with radiosensitivity of brain metastases, we investigated the association of S100A9 with prognosis in patients with brain metastases receiving standard care, including radiotherapy, in a clinical population .
By analyzing clinical specimens and follow-up data of patients with lung cancer (n=22), breast cancer (n=42), and melanoma (n=34) brain metastases, the researchers found that S100A9 expression in lung brain metastases specimens Levels were correlated with tumor recurrence time after whole brain radiotherapy, and S100A9 expression levels in breast cancer and melanoma brain metastases specimens were significantly correlated with survival . This strongly suggests the value of S100A9 as a predictive radiosensitivity marker.
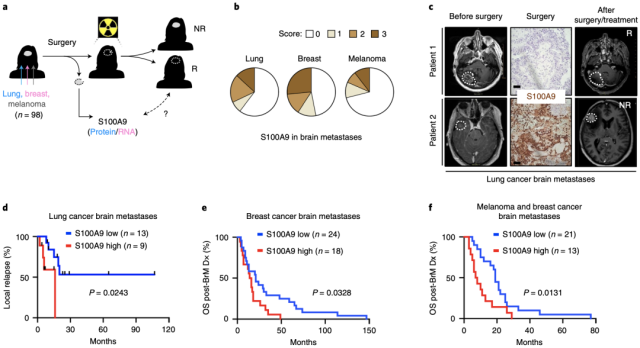
Brain tumor specimen S100A9 level can be used as a predictor of radiosensitivity markers
Considering that some patients with brain metastases have lost the opportunity for surgery and cannot obtain intracranial tumor specimens to evaluate the expression level of S100A9, the researchers also studied the relationship between the level of S100A9 in blood and the radiosensitivity of patients.
By analyzing blood samples and clinical data from 71 patients with brain metastases, they found that S100A9 levels in blood can also be used to predict the prognosis of patients receiving standard treatment (including radiotherapy) , although larger clinical studies are needed to determine this. verify.
In order to allow those patients with high S100A9 levels to enjoy the same benefits of radiation therapy, the researchers could not help but wonder, can this radiation resistance be reversed?
Fortunately, the existing RAGE inhibitor FPS-ZM1 penetrates the blood-brain barrier very well [5]. After using FPS-ZM1 in mice with lung cancer brain metastases and breast cancer brain metastases, the researchers found that tumors that were originally resistant to radiotherapy became sensitive to the treatment.
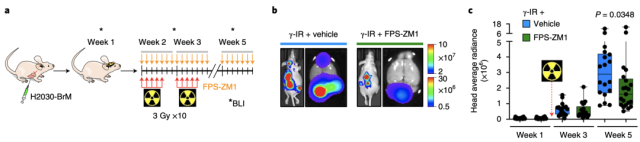
RAGE inhibitor FPS-ZM1 reverses tumor radioresistance
Overall, Valiente’s team found that S100A9 mediates radiotherapy resistance in brain metastases and can be used as a potential biomarker to predict the sensitivity of patients to radiotherapy, so as to achieve the purpose of personalized treatment .
It is understood that researchers have initiated a prospective multicenter study to evaluate the predictive value of S100A9 for radiosensitivity.
More importantly, this radiotherapy resistance can be reversed by RAGE inhibitors, and RAGE inhibitors have entered clinical trials for the treatment of Alzheimer’s disease [6].
In the near future, the drug may actually be used as a radiosensitizer in clinical use to improve the prognosis of patients with brain metastases.
references
1.Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL: Current approaches to the management of brain metastases. Nat Rev Clin Oncol 2020, 17(5):279-299.
2.Mulvenna P, Nankivell M, Barton R, Faivre-Finn C, Wilson P, McColl E, Moore B, Brisbane I, Ardron D, Holt T et al: Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016, 388(10055):2004-2014.
3.Monteiro C, Miarka L, Perea-García M, Priego N, García-Gómez P, Álvaro-Espinosa L, de Pablos-Aragoneses A, Yebra N, Retana D, Baena P et al: Stratification of radiosensitive brain metastases based on an actionable S100A9/RAGE resistance mechanism. Nature Medicine 2022.
4.Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob L, Patwa R, Shah H, Xu K et al: Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 2016, 533(7604):493-498.
5.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ et al: A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 2012, 122(4):1377-1392.
6.Burstein AH, Sabbagh M, Andrews R, Valcarce C, Dunn I, Altstiel L: Development of Azeliragon, an Oral Small Molecule Antagonist of the Receptor for Advanced Glycation Endproducts, for the Potential Slowing of Loss of Cognition in Mild Alzheimer’s Disease. J Prev Alzheimers Dis 2018, 5(2):149-154.
Solving the Mystery of Radiotherapy Resistance of Brain Metastasis Cancer Cells
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



