QCCs may play an important role in the recurrence of triple-negative breast cancer
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
QCCs may play an important role in the recurrence of triple-negative breast cancer
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
QCCs may play an important role in the recurrence of triple-negative breast cancer
Triple-negative breast cancer (TNBC) is the most aggressive type of breast cancer. Because it does not express hormone receptors and HER2, targeted therapy cannot be used. For a long time, TNBC patients can only receive surgery and radiotherapy and chemotherapy.
The emergence of immune checkpoint inhibitor therapy has brought new hope for TNBC patients, however, clinical trials have found that PD-1 inhibitors combined with chemotherapy can only benefit a small proportion of TNBC patients [1,2]. Therefore, it is crucial to explore the mechanism of TNBC resistance to immune checkpoint inhibitor therapy, which will provide a reference for improving the effect of immunotherapy.
Recently, a research team led by Professor Judith Agudo of Dana-Farber Cancer Center published important research results in the journal Cell [3].
They found that there is a group of quiescent tumor cells (QCCs) in TNBC that resist T cell killing. These QCCs, together with immunosuppressive fibroblasts and dysfunctional DC cells, form a microenvironment that resists T cell infiltration and killing by activating HIF1a. Thereby surviving immunotherapy and causing tumor recurrence.
The results of this study unravel the mystery of breast cancer immune escape, highlight the important role of QCCs in breast cancer immune escape, and suggest that targeting and clearing QCCs may be an effective way to solve the immune resistance problem of TNBC.

Previous studies have found that some genes play a role in tumor cell resistance to immunotherapy [4,5], but these studies cannot explain how tumor cells create an immunosuppressive microenvironment; in addition, sequencing can be used to find However, there are a large number of heterogeneous cell populations in tumor tissues, which greatly increases the difficulty of finding tumor resistance mechanisms. For example, cancer cells that lose tumor antigens and tumor antigen-positive cancer cells take different approaches in escaping from the immune system.
In this study, Professor Agudo’s team wanted to explore how cancer cells expressing tumor antigens escape the immune system. Therefore, they inoculated mice with the TNBC cell line 4T07 expressing GFP or mCherry, and transfused the mice with PD-1-/-Jedi T cells that specifically recognize GFP (the PD-1 knockout was to mimic PD-1 inhibitor therapy).
Since the TCR of Jedi T cells can only specifically recognize GFP but not mCherry . Therefore, this experimental model can be used to explore how tumor cells that continuously express tumor-specific antigens (GFP+ tumor cells) escape the attack of tumor antigen-specific T cells (Jedi T cells).
The research team analyzed the ratio of GFP+ to mCherry+ tumor cells in the tumor on the 5th day after transfusion of Jedi T cells, and found that Jedi T cells killed most of the GFP+ tumor cells, but some GFP+ tumor cells still escaped the killing of T cells.
Immunofluorescence staining revealed that these escaped GFP+ tumor cells formed a cell cluster within which the level of T cell infiltration was reduced by a factor of two.
This suggests that the surviving GFP+ tumor cell clusters can prevent T cell infiltration, which may be one of the reasons for their resistance to immunotherapy.
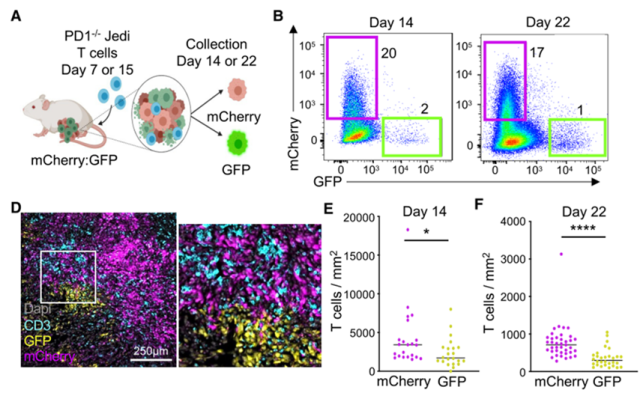
Surviving GFP+ tumor cells clustered after Jedi T cell challenge
Next, Prof. Agudo’s team analyzed the transcriptome differences between surviving GFP+ tumor cells and control mCherry+ tumor cells.
They found that genes related to the cell cycle were significantly downregulated in GFP+ tumor cells compared to mCherry+ tumor cells. EdU (thymidine analog 5-ethynyl-20-deoxyuridine) incorporation experiments showed that these GFP+ tumor cells that survived Jedi T cell challenge were in a state of cell cycle arrest (termed quiescent tumor cells, QCCs).
So are these QCCs more resistant to T cell killing? The research team transferred tdTomato-p27K into tumor cells, which enables QCCs to be labeled with tdTomato.
After transfusion of PD-1-/- Jedi T cells into tumor-bearing mice, it was found that most of the surviving GFP+ tumor cells were tdTomato+ compared with the control non-immunogenic miRFP670+ tumor cells, which indicated that QCCs had no effect on T cells. The killing has a stronger resistance.
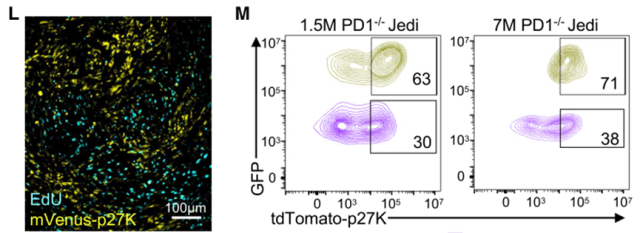
GFP+ tumor cells that survived Jedi T cell challenge are in a cell cycle quiescent state and are more resistant to T cell killing
The above results show that in mouse models, QCCs are the main force of immunotherapy resistance. Does this phenomenon also exist in human patients?
By analyzing the histopathological samples of breast cancer patients and transcriptome sequencing, the research team found that most of the tumor cells in contact with T cells in the tumor tissue were Ki67+ proliferating cells, and p27+ QCCs rarely contacted T cells ; Cluster analysis showed that tumor cells in immunotherapy-responsive patients were more enriched in DNA replication-related pathways than in treatment non-responders, suggesting that they were in a non-quiescent state.
This correlation suggests that QCCs may be associated with poor immunotherapy outcomes in breast cancer patients.
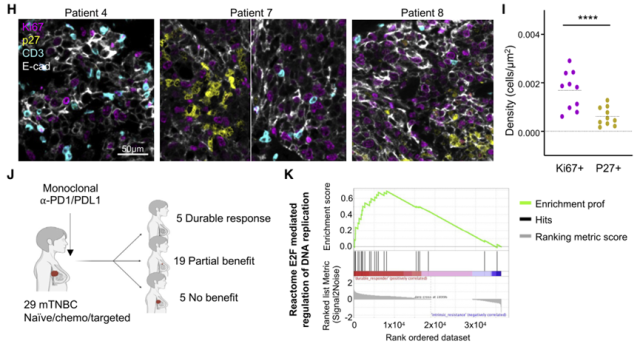
T cell infiltration around resting tumor cells is less in breast cancer patients’ tumor tissue, and tumor cells are in a cell cycle active state in patients who respond to immunotherapy
To explore the drug resistance mechanism of QCCs, the research team performed RNA sequencing on QCCs and non-quiescent tumor cells.
Data analysis showed that QCCs up-regulated hypoxia and glucose metabolism-related pathway genes.
Immunofluorescence staining showed that p27K+ QCCs co-localized with hypoxic probes, while T cells rarely infiltrated into hypoxic areas; GFP+ tumor cells that survived Jedi T cell therapy were also located in hypoxic areas.
These data suggest that QCCs in breast cancer are predominantly located in hypoxic microenvironments with less T cell infiltration.
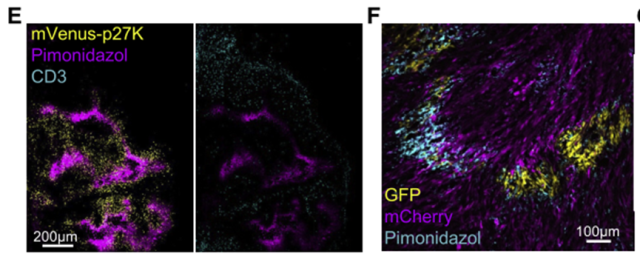
The area where the QCCs are located is markedly hypoxic and has less T cell infiltration
Next, to analyze the mechanism by which QCCs suppress T cell function at the single-cell level, Prof.
Agudo’s team isolated infiltrating cells inside and outside the QCCs cell clusters and performed single-cell transcriptome sequencing.
Through data analysis, it was found that there were a large number of immunosuppressive fibroblasts in the QCCs cell clusters , which explained why the number of infiltrating T cells in the QCCs area was low.
The analysis of the infiltrating T cells inside and outside the QCCs cell cluster found that the CD8+ T cells infiltrating in the hypoxic QCCs cell cluster were more deeply depleted and had weaker killing ability.
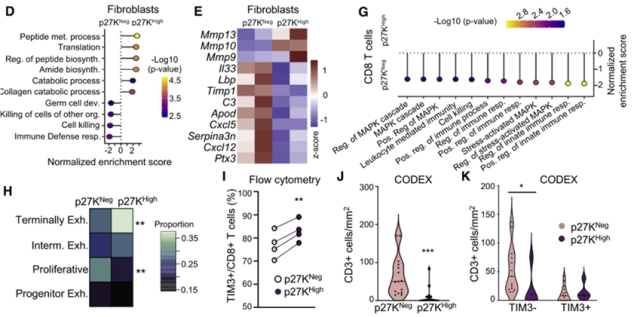
There are a large number of immunosuppressive fibroblasts in the QCCs cell cluster, the number of infiltrating T cells is significantly lower than that outside the QCCs cell cluster, and the infiltrating T cells in the QCCs cell cluster are more depleted
What groups of cells are contributing to T cell dysfunction? By scoring the hypoxia characteristics of each infiltrating cell subset in QCCs, the research team found that DC cells expressed abundant hypoxia-related genes, and down-regulated genes that promote T cell activation, such as MHC I and MHC II, IL-12, CD80/86, It is shown that the ability of these DC cells to promote T cell activation is greatly attenuated, which may be an important reason for the dysfunction of T cells infiltrating inside the QCCs cell clusters.
However, Professor Agudo’s team found through hypoxia induction experiments that hypoxia does not directly impair the ability of DC cells to activate T cells, so the research team turned their attention to QCCs themselves.
To explore whether HIF1a-expressing QCCs shape an immunosuppressive microenvironment, impair the ability of DCs to activate T cells and thereby suppress T cell immune responses. To this end, they constructed breast cancer cell lines expressing activated HIF1a ( HIF1a STBL ).
Tumor-bearing experiments showed that compared with WT tumors, HIF1a STBL -expressing tumors had fewer infiltrating T cells and deeper depletion; MHC II expression was significantly down-regulated on intratumoral DC cells, which was consistent with what was observed within the QCCs cell clusters. Basically the same.
Furthermore, the research team inoculated WT or HIF1a STBL -expressing GFP+ tumor cells mixed with mCherry+ tumor cells subcutaneously into mice.
It was found that compared to the mCherry+:GFP+ tumor group, mice inoculated with mCherry+:GFP+ HIF1a STBL tumor cells had fewer intratumoral infiltrating T cells, and T cells were less accessible to GFP+ tumor cells, and the number of surviving GFP+ tumor cells increased Several times, it can be seen that HIF1a can promote the immune escape of tumor cells.
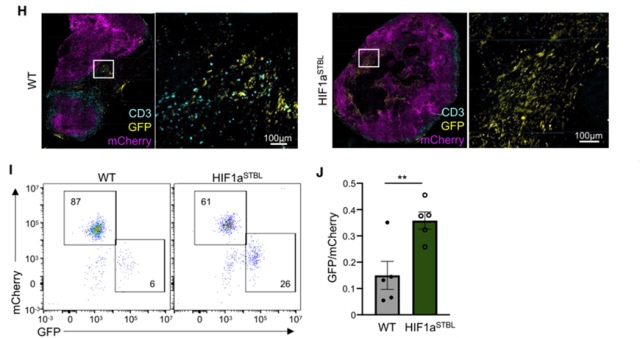
Tumor cells overexpressing HIF1aSTBL can inhibit T cell infiltration and escape T cell attack
Based on the above data, it can be seen that QCCs create an immunosuppressive microenvironment through high expression of HIF1a and escape the pursuit of tumor antigen-specific CD8+ T cells. So can knocking out HIF1a in tumor cells promote T cell killing of tumors?
The experimental results showed that after knocking out HIF1a in tumor cells, the number of infiltrating T cells in the tumor was significantly increased, the degree of exhaustion was reduced, and the tumor volume was significantly reduced.
This suggests that targeting HIF1a in tumors can promote anti-tumor immune responses and inhibit tumor growth.
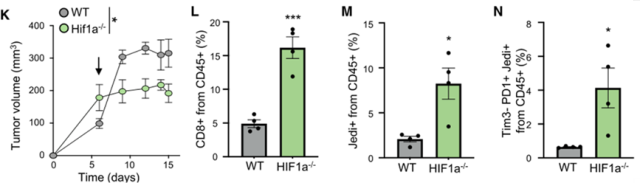
Knockdown of HIF1a in tumor cells significantly enhances antitumor immune responses
Collectively, this study sheds light on the mechanism by which tumor antigen-positive cancer cells escape immune: they aggregate into clusters and summon fibroblasts to build a barrier for them; together they enter a quiescent state and overexpress HIF1a, allowing the immune system to Sentinel-DC cells are dysfunctional, which in turn suppresses T-cell immune responses. These efforts allowed them to escape T cells and make a comeback after immunotherapy.
At the same time, the results of this study also suggest that eradication of QCCs is the key to improving the effect of TNBC immunotherapy and preventing tumor recurrence.
references:
1. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind , phase 3 clinical trial. Lancet. 2020;396(10265):1817-1828. doi:10.1016/S0140-6736(20)32531-9
2. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med. 2018;379(22):2108-2121. doi:10.1056/NEJMoa1809615
3. Baldominos P, Barbera-Mourelle A, Barreiro O, et al. Quiescent cancer cells resist T cell attack by forming an immunosuppressive niche [published online ahead of print, 2022 Apr 15]. Cell. 2022;S0092-8674(22) 00343-9. doi:10.1016/j.cell.2022.03.033
4. Pan D, Kobayashi A, Jiang P, et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science. 2018;359(6377):770-775. doi:10.1126/science.aao1710
5. Patel SJ, Sanjana NE, Kishton RJ, et al. Identification of essential genes for cancer immunotherapy. Nature. 2017;548(7669):537-542. doi:10.1038/nature23477
QCCs may play an important role in the recurrence of triple-negative breast cancer
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



