Seven patients die: MacroGenics closes its B7-H3-targeted solid tumor clinical trial
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Seven patients die: MacroGenics closes its B7-H3-targeted solid tumor clinical trial
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Seven patients die: MacroGenics closes its B7-H3-targeted solid tumor clinical trial.
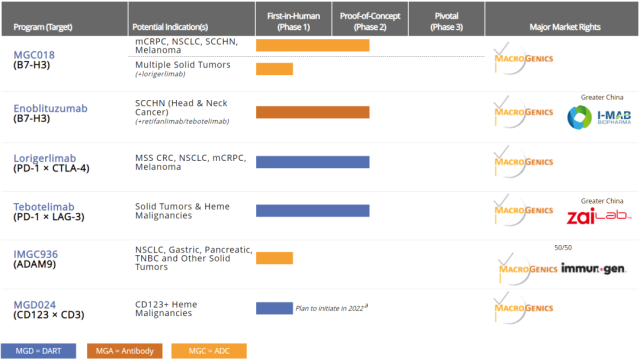
Recently, the B7-H3- targeted monoclonal antibody developed by MacroGenics , Enoblituzumab, in the treatment of head and neck squamous cell carcinoma in the phase 2 clinical trial, 7 patients died, MacroGenics announced the closure of this clinical trial.
This is also the second setback for MacroGenics in its three R&D pipelines for the B7-H3 target. In 2020, the company stopped the development of its B7-H3×CD3 bispecific antibody .
This time, the development of the B7-H3 monoclonal antibody was stopped . The clinical trial of B7-H3 ADC is still advancing and is in Phase 2 clinical phase.

In this phase 2 clinical trial of Enoblituzumab combined with PD-1 mAb in the treatment of head and neck squamous cell carcinoma, 7 of 62 patients participating in the clinical trial died (related mortality reached 11.3%) , these patients died May be related to bleeding events.
Bleeding is a known risk for head and neck squamous cell carcinoma, but the incidence of this risk should be lower than in this phase 2 clinical trial.
MacroGenics Chief Executive Officer Scott Koenig said in a statement that he was surprised by the multiple deaths in the clinical trial, as previous early-stage clinical trials had not seen such events.
In a phase 3 clinical trial of Bevacizumab developed by Roche in the treatment of head and neck squamous cell carcinoma, the bleeding-related mortality rate of patients in the treatment group was 3.6%, while the blood-related mortality rate of patients in the previous clinical trial of MacroGenics was less than 1 %.
The data prompted MacroGenics to halt the clinical trial, although it cannot yet prove that the patient’s death was related to the treatment.
In addition to the monoclonal antibody Enoblituzumab, MacroGenics has five R&D pipelines, including two ADC drugs ( B7-H3 target and ADAM9 target respectively ) , and three bispecific antibodies (PD-1 × CTLA- 4. PD-1×LAG-3, CD123×CD3) .
References :
https://macrogenics.com/pipeline/
Seven patients die: MacroGenics closes its B7-H3-targeted solid tumor clinical trial
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



