Blood tests for Alzheimer’s disease are different for men and women!
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Molecular Psychiatry: Blood tests for Alzheimer’s disease are different for men and women!
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Molecular Psychiatry: Blood tests for Alzheimer’s disease are different for men and women!
Since ancient times, beauty has faded, and aging is a life course that everyone cannot escape.
In the face of aging, we all hope to age gracefully and calmly. However, there is one disease that casts a haze on this unbeautiful aging, and it is Alzheimer’s disease (AD), the most predominant type of dementia.
Several studies have found higher levels of AD pathological biomarkers in the brain and cerebrospinal fluid of women compared with men, and stronger associations with cognitive decline and neurodegeneration. Whether this correlation also exists for AD biomarkers in blood remains unknown .
Recently, a team led by Sarah J. Banks of the Department of Neuroscience at the University of California, San Diego, published a new study in the journal Molecular Psychiatry [1].
The Banks team found that although men and women had similar plasma p-tau181 levels, higher plasma p-tau181 levels in women were associated with lower brain glucose metabolism, brain beta-amyloid (Aβ) and entorhinal cortical tau compared with men Higher deposition, higher cerebrospinal fluid (CSF) p-tau181, and faster cognitive decline were associated .
The results of this study suggest that gender may influence the clinical interpretation of plasma p-tau181 concentrations .
If this result is reproducible in a larger population sample, this finding will have important implications for plasma p-tau181 as an AD biomarker and screening tool for preventive and therapeutic clinical trials.

Screenshot of the paper’s homepage
AD was first discovered in 1906 by German psychiatrist and pathologist Alois Alzheimer, a neurodegenerative disease with a slow onset and progressive deterioration over time.
Currently, AD has become a global healthcare challenge, affecting more than one in nine adults over the age of 65 in the United States alone [2].
Currently, the etiology of AD remains unknown, and several different hypotheses attempt to explain the etiology of AD.
A dominant etiological explanation is the Aβ hypothesis, proposed in 1991, that Aβ deposition in the brain may be the root cause of AD [3].
In addition, the Tau protein hypothesis believes that abnormal Tau protein is the main cause of AD disease development [4].
Although Aβ positron emission tomography and cerebrospinal fluid detection have greatly improved the accuracy of AD diagnosis and prognosis [5-7], these methods are not only expensive but also invasive, making them largely difficult to use in clinical practice on the application.
Therefore, biomarkers in the blood of AD patients may be potential surrogates [8].
Recent studies have shown that plasma p-tau181 can identify individuals with AD, discriminating between Aβ-positive and Aβ-negative individuals with an accuracy comparable to established AD biomarker prediction methods [9].
Furthermore, higher plasma p-tau181 at baseline was associated with higher levels of cortical tau by [ 18 F] Flortaucipir positron emission tomography (FTP-PET) [10].
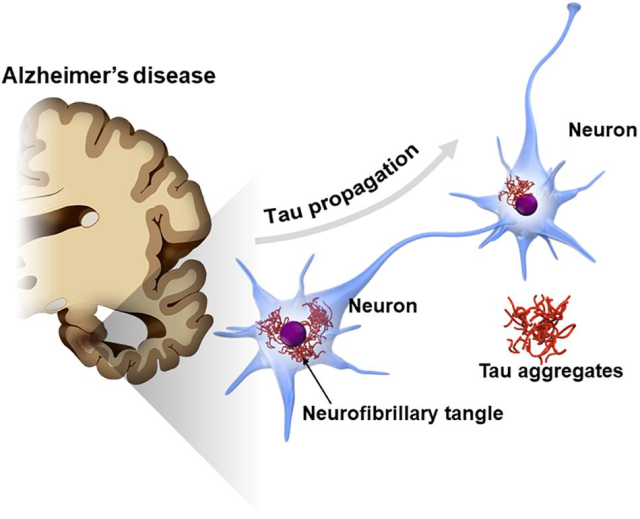
Source[11]
Not only that, there is growing evidence that women with AD gradients have higher levels of CSF and tau in the brain compared to men [12].
An autopsy study found that every 1-unit increase in tau pathology was associated with a 13-fold increase in the odds of clinical AD in women and only a 1.4-fold increase in men [13].
Another study showed that in individuals with higher CSF tau levels, female hippocampal atrophy was more pronounced and executive function declined more rapidly than males [14].
Combined with previous evidence showing a strong association between tau protein deposition and AD clinical manifestations [15], an increased number of tau pathologies and increased susceptibility to tau pathology in women may lead to more dramatic cognitive decline [16].
However, it is unclear whether similar sex differences exist in plasma p-tau181 and its association with cognitive decline, AD risk, and other AD biomarkers .
In view of this, the Banks team attempted to answer the following three questions:
① whether the cross-sectional and longitudinal levels of plasma p-tau181 differ by gender in individuals with cognitive and clinical gradient profiles;
② whether gender alters the relationship between plasma p-tau181 and CSF p-tau181 tau181, Aβ deposition, glucose metabolism and tau deposition in the brain;
③ Whether gender changes the effect of baseline plasma p-tau181 on cognitive decline and progression to AD dementia.
To answer these questions, the Banks team used the AD Neuroimaging Initiative (ADNI) database.
Data for this analysis were obtained from the Laboratory of Neuroimaging (LONI) database (ida.loni.usc.edu) in June 2021.
The study sample included 1060 participants, of whom 494 were women (46.7%) and 566 were men (53.4%).
Of all participants, 359 (33.9%) were cognitively normal, 516 (48.7%) were diagnosed with mild cognitive impairment, 185 (17.5%) were diagnosed with dementia, and 560 (52.8%) were Aβ positive.
The study sample was predominantly white (92.6%), with a mean age of 73.8 years and an average of 16.2 years of education.
Compared with men, women in the sample were younger, had fewer years of education, had higher levels of CSF p-tau181 and brain glucose metabolism, were more likely to be cognitively normal, and were less likely to have mild cognitive impairment or dementia (Table 1 ).
To account for gender differences in diagnostic status, we additionally adjusted for the effect of diagnosis in subsequent analyses.
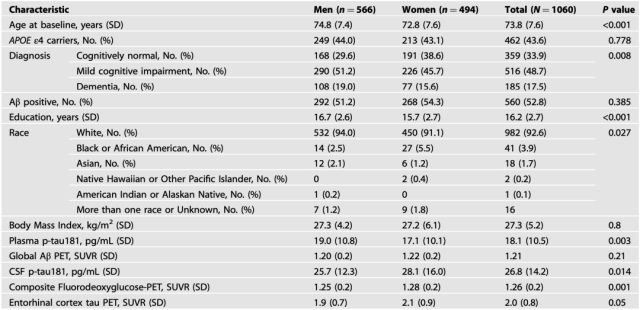
Table 1 Baseline demographic and clinical sample characteristics by sex
Results of the study showed that no significant differences in baseline plasma p-tau181 levels were observed between males and females in the entire cohort and any stratified group, and the rate of change in plasma p-tau181 over time in the entire cohort and stratified group There are no gender differences. That is, plasma p-tau181 concentrations were similar between the sexes .
So what is the difference between the sexes in the process of AD?
The Banks team found that while plasma p-tau181 concentrations were similar between the sexes, the phenotypic biomarker profile was worse in women .
The phenotypic biomarker profile was associated with elevated plasma p-tau181 concentrations in women compared with men.
Specifically, higher plasma p-tau181 levels in women were associated with greater cortical Aβ deposition, higher concentrations of p-tau181 in the CSF, greater entorhinal cortical tau deposition, and lower brain glucose metabolism compared with men .
In addition, compared with men, women experienced faster cognitive decline and a greater risk of transitioning to dementia, which was associated with higher baseline plasma p-tau181 levels .
Notably, these gender differences were seen in Aβ-positive participants and individuals with mild cognitive impairment, but not in dementia patients .
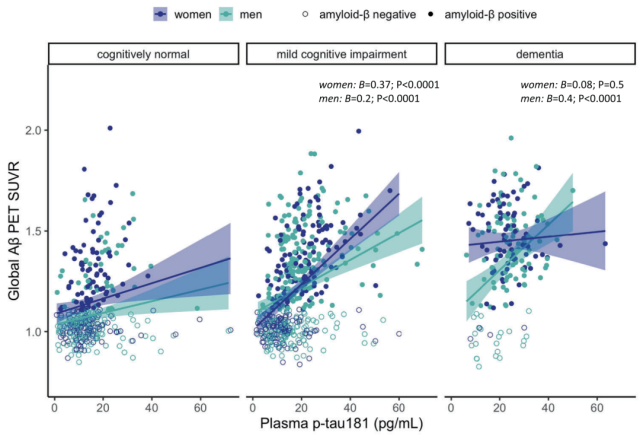
Gender modulates the association of plasma p-tau181 with brain Aβ deposition
The Banks team also found that Aβ-positive women were associated with higher rates of dementia associated with higher baseline plasma p-tau181 compared with Aβ-positive men.
This finding is consistent with previous studies. For example, studies have shown that in the presence of elevated tau load in the brain and CSF, women exhibit faster trajectories of cognitive decline and higher rates of clinical AD diagnosis compared with men [13, 14]; and Studies have shown that although women exhibit greater cognitive resilience in early pathological stages of AD, they experience a faster clinical decline in more advanced stages of pathology [17].
Therefore, it is likely that some of the women in the Aβ-positive and mild cognitive impairment groups in this study broke through a critical point on their AD trajectory, and clinical symptoms began to accelerate .
Overall, the Banks team’s findings suggest that gender may influence the clinical interpretation of plasma p-tau181 concentrations .
Although plasma p-tau181 levels were similar in men and women, higher plasma p-tau181 levels in women were associated with lower brain glucose metabolism, more Aβ and entorhinal cortex tau deposition, and higher CSF p-tau181 compared with men It is associated with a faster decline in cognitive ability.
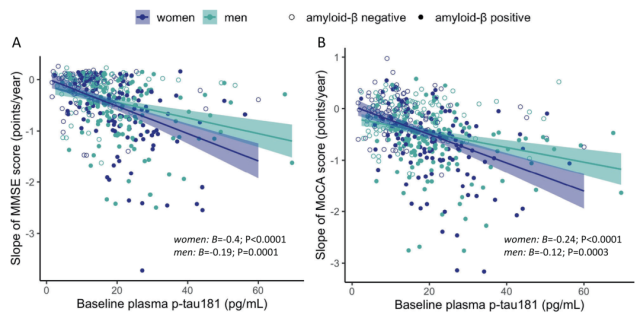
Gender modulates the association of plasma p-tau181 with cognitive changes
It should be pointed out that this study has certain limitations.
The study sample consisted primarily of non-Hispanic white participants, which limits the generalizability of the findings.
Previous studies have shown that gender differences in clinical AD trajectories differ between racial and ethnic groups [18], and future studies should investigate whether gender affects the clinical interpretation of plasma p-tau181 concentrations in samples from different populations .
In addition, the present study of entorhinal cortex tau deposition was obtained by PET scan, but several years after the measurement of plasma p-tau181.
Although the researchers adjusted their models for this time lag, they were unable to draw conclusions about the temporal nature of the relationship between plasma p-tau181 levels and cortical tau deposition.
If the conclusions of this study are further confirmed, in the future, perhaps by detecting potential sex differences in plasma p-tau181, sex-specific guidelines could be developed, which could also be used as a screening tool in clinical trials, and as a biomarker for the prevention of AD.
references
[1]Tsiknia AA, Edland SD, Sundermann EE, et al. Sex differences in plasma p-tau181 associations with Alzheimer’s disease biomarkers, cognitive decline, and clinical progression [published online ahead of print, 2022 Jun 29]. Mol Psychiatry. 2022 ;10.1038/s41380-022-01675-8. doi:10.1038/s41380-022-01675-8
[2] alzheimers-facts-and-figures.pdf. 2021. https://www.alz.org/media/documents/alzheimers-facts-and-figures.pdf
[3] Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12(10):383-388. doi:10.1016/0165-6147(91)90609-v
[4] Mudher A, Lovestone S. Alzheimer’s disease-do tauists and baptists finally shake hands?. Trends Neurosci. 2002;25(1):22-26. doi:10.1016/s0166-2236(00)02031-2
[5] Skillbäck T, Farahmand BY, Rosén C, et al. Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain. 2015;138(Pt 9):2716-2731. doi:10.1093/brain/awv181
[6]Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study [published correction appears in Lancet Neurol. 2006 Apr;5(4):293]. Lancet Neurol. 2006;5(3):228-234. doi:10.1016/S1474-4422(06)70355-6
[7] Hanseeuw BJ, Betensky RA, Jacobs HIL, et al. Association of Amyloid and Tau With Cognition in Preclinical Alzheimer Disease: A Longitudinal Study [published correction appears in JAMA Neurol. 2019 Aug 1;76(8):986]. JAMA Neurol. 2019;76(8):915-924. doi:10.1001/jamaneurol.2019.1424
[8]Molinuevo JL, Ayton S, Batrla R, et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018;136(6):821-853. doi:10.1007/s00401-018-1932-x
[9]Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho-tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Mol Psychiatry. 2021;26(2):429-442. doi: 10.1038/s41380-020-00923-z
[10]Moscoso A, Grothe MJ, Ashton NJ, et al. Time course of phosphorylated-tau181 in blood across the Alzheimer’s disease spectrum [published correction appears in Brain. 2021 Jul 28;144(6):e57]. Brain. 2021 ;144(1):325-339.doi:10.1093/brain/awaa399
[11] Takeda S. Tau Propagation as a Diagnostic and Therapeutic Target for Dementia: Potentials and Unanswered Questions. Front Neurosci. 2019;13:1274. Published 2019 Dec 13. doi:10.3389/fnins.2019.01274
[12] Shokouhi S, Taylor WD, Albert K, Kang H, Newhouse PA; Alzheimer’s Disease Neuroimaging Initiative. In vivo network models identify sex differences in the spread of tau pathology across the brain. Alzheimers Dement (Amst). 2020;12( 1): e12016. Published 2020 Apr 9. doi: 10.1002/dad2.12016
[13] Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685-691. doi:10.1001 /archpsyc.62.6.685
[14] Koran MEI, Wagener M, Hohman TJ; Alzheimer’s Neuroimaging Initiative. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging Behav. 2017;11(1):205-213. doi:10.1007/s11682-016 -9523-8
[15] Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139(Pt 5):1551-1567. doi:10.1093/brain/aww027
[16] Buckley RF, Scott MR, Jacobs HIL, et al. Sex Mediates Relationships Between Regional Tau Pathology and Cognitive Decline. Ann Neurol. 2020;88(5):921-932. doi:10.1002/ana.25878
[17] Sundermann EE, Biegon A, Rubin LH, et al. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology. 2016;86(15):1368-1376. doi:10.1212/WNL. 0000000000002570
[18]Avila JF, Vonk JMJ, Verney SP, et al. Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimers Dement. 2019;15(12):1516-1523. doi:10.1016/j .jalz.2019.04.006
Molecular Psychiatry: Blood tests for Alzheimer’s disease are different for men and women!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



