Gut bacteria enhance the effect of cancer chemotherapy through metabolites
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Gut bacteria enhance the effect of cancer chemotherapy through metabolites
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Nature Heavy: Gut bacteria enhance the effect of cancer chemotherapy through metabolites, laying the foundation for dietary intervention in cancer
Pancreatic cancer is a highly malignant tumor of the digestive tract that is difficult to diagnose and treat. Among them, pancreatic ductal adenocarcinoma (PDAC) accounts for more than 95% of all pancreatic cancers.
In recent years, the morbidity and mortality of pancreatic cancer have increased significantly. The early diagnosis rate of pancreatic cancer is not high, and it is often discovered at an advanced stage when the cancer cells have already spread.
The 5-year survival rate of pancreatic cancer is less than 11%. It is one of the malignant tumors with the worst prognosis and is also known as the ” king of cancer “.
For metastatic pancreatic ductal adenocarcinoma (mPDAC) , combined chemotherapy is the current standard of care, however, half of patients who do not respond to chemotherapy suffer and die within weeks.
The difference between response and non-response to chemotherapy was poorly explained by genetic alterations in patients, making environmental factors, including the composition of the gut microbiome, a potential contributor to chemotherapy efficacy.
Gut microbiota have previously been shown to induce responses to immunotherapy in patients with melanoma and can be modulated by diet.
However, it remains unclear whether and how the gut microbiota or diet can affect treatment outcomes in metastatic pancreatic ductal adenocarcinoma (mPDAC) .
Recently, German researchers published a research paper entitled: Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer in the top international academic journal Nature .
This study found that indole-3-acetic acid (3-IAA), a tryptophan metabolite produced by two gut bacteria (Bacteroides fragilis, Bacteroides polymorpha), was effective in pancreatic ductal adenocarcinoma (PDAC) responding to chemotherapy.
Levels were better in patients, and chemotherapy effects were enhanced by direct supplementation with 3-IAA or a diet high in tryptophan.
This study explored the impact of gut microbiota on chemotherapy for pancreatic ductal adenocarcinoma, uncovered ways in which gut bacteria can positively influence cancer treatment, and provides impetus for dietary intervention during cancer patient treatment.
The research team recruited 30 patients with metastatic pancreatic ductal adenocarcinoma (mPDAC) , 23 of whom had not received antibiotics, who provided enough samples to analyze their gut microbiota before starting chemotherapy.
The results showed differences in the gut microbiomes of pancreatic cancer patients who responded to chemotherapy (11 people) and those who did not (12 people) .
They also found that when mice with pancreatic cancer that did not respond to chemotherapy were depleted of their gut microbiota, and then transplanted with the gut microbiota of mice with pancreatic cancer that responded to chemotherapy, they began to respond well to chemotherapy after receiving the transplant.
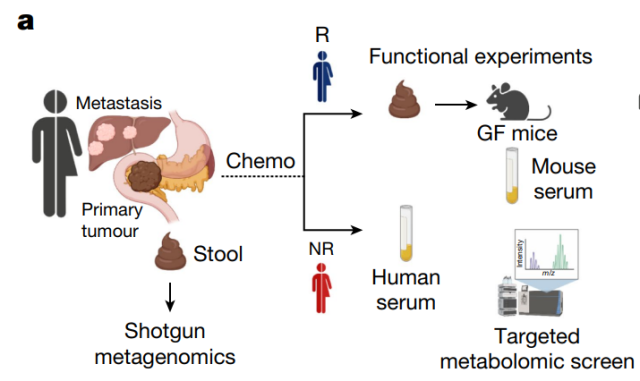
To better understand how the gut microbiome plays a role in chemotherapy effects, the research team collected blood samples from patients who responded well to chemotherapy and those who did not.
They found that patients who responded better had higher levels of the molecule indole-3-acetic acid (3-IAA) , a metabolite of tryptophan , and further research showed that these 3-IAAs were produced by two gut bacteria Produced by Bacteroides fragilis and Bacteroides thetaiotaomicron .
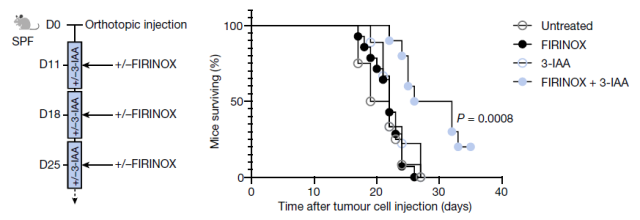
The research team tried two methods to increase 3-IAA levels. The research team added 3-IAA directly to the food eaten by cancer mouse models, and the results showed that they responded more strongly to chemotherapy. Since 3-IAA is a metabolite of tryptophan, the research team also fed cancer mouse models a high-tryptophan diet, but tryptophan impairs the antitumor immune response, which promotes tumor growth.
Therefore, the research team tested high-tryptophan diet intervention for different lengths of time, and the results showed that 14 days of high-tryptophan diet can promote tumor growth, while 4 days can increase serum 3-IAA content, when combined with chemotherapy , can reduce tumor size, and the serum level of 3-IAA is negatively correlated with tumor size.
These experimental data demonstrate that the levels of the microbially derived tryptophan metabolite 3-IAA are increased in human and mouse sera of metastatic pancreatic ductal adenocarcinoma (mPDAC) responding to chemotherapy , and that 3-IAA levels can be expressed by Dietary tryptophan to regulate. Furthermore, 3-IAA was able to regenerate even chemotherapy-resistant mPDACs to be responsive.
The research team further explored why higher levels of 3-IAA help improve the effectiveness of chemotherapy.
Through loss-of-function and gain-of-function experiments, the research team found that the efficacy of 3-IAA combined with chemotherapy was mediated by neutrophil -derived myeloperoxidase (MPO) .
Oxidation of 3-IAA by myeloperoxidase, combined with chemotherapy-induced downregulation of the reactive oxygen species (ROS) degrading enzymes glutathione peroxidase 3 (GPX3) and glutathione peroxidase 7 (GPX7) .
All of these lead to the accumulation of ROS and the downregulation of autophagy in cancer cells, which impairs their metabolic fitness and ultimately suppresses the proliferation of cancer cells.
The research team concluded that 3-IAAs metabolized by B. fragilis and B. polymorpha in the gut microbiota are transported through the blood to distant tumors, where they exert enhanced chemotherapy by stimulating immune cells (neutrophils ) . to help fight cancer.
The research team further observed a significant correlation between 3-IAA levels and chemotherapy efficacy in two independent human pancreatic ductal adenocarcinoma (PDAC) cohorts.
Collectively, this study identified a gut microbiome-derived metabolite, indole-3-acetic acid (3-IAA) , with clinical implications in pancreatic ductal adenocarcinoma (PDAC) Motivation was provided to consider nutritional interventions during patient care.
Paper link :
https://www.nature.com/articles/s41586-023-05728-y
Gut bacteria enhance the effect of cancer chemotherapy through metabolites
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



