How to play a synergistic role between T cells and NK cells?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
How to play a synergistic role between T cells and NK cells?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
How to play a synergistic role between T cells and NK cells?
Tumor heterogeneity is one of the major challenges facing cancer therapeutic approaches, including immunotherapy. Heterogeneity can arise through the acquisition of new mutations, resulting in treatment-resistant tumor subclones.
Furthermore, cancer cells can adapt to therapeutic stress by changing their cellular state. Thus, we are faced with the task of how to actively design immunotherapies that prevent the selection of drug-resistant tumor cells.
T cells and natural killer ( NK ) cells have complementary roles in tumor immunity.
These lymphocyte populations use different recognition strategies to identify cancer cells, and NK cells can kill cancer cells that escape the recognition of CD8+ T cells.
The dual attack of T cells and NK cells therefore provides an opportunity to deepen the impact of immunotherapy.
Recent studies have also shown that NK cells play an important role in recruiting dendritic cells to tumors, thereby enhancing the induction of CD8+ T cell responses, while IL-2 secreted by T cells activates NK cells.
Interestingly, T cells and NK cells also share several important inhibitory and activating receptors that can serve as targets for enhanced T-cell and NK-cell immunity.
These inhibitory receptor ligand systems include CD161-CLEC2D, TIGIT-CD155 and NKG2A/CD94-HLA-E.
In addition, new therapeutic strategies based on inhibiting and activating cytokines can also exert the synergistic effect of T cells and NK cells.
These therapies based on the synergistic effect of T cells and NK cells will profoundly affect the current cancer immunotherapy.
Molecular logic of tumor cell recognition by T cells and NK cells
T cells and NK cells fundamentally differ in the molecular mechanisms of tumor cell recognition.
T cells recognize tumor cells through TCR- mediated MHC- binding peptide antigens.
All critical functions of T cells are under the control of TCRs , including cytotoxicity, cytokine production, and proliferation.
Therefore, tumors can escape the effects of CD8+ T cells by downregulating or losing MHC-I expression.
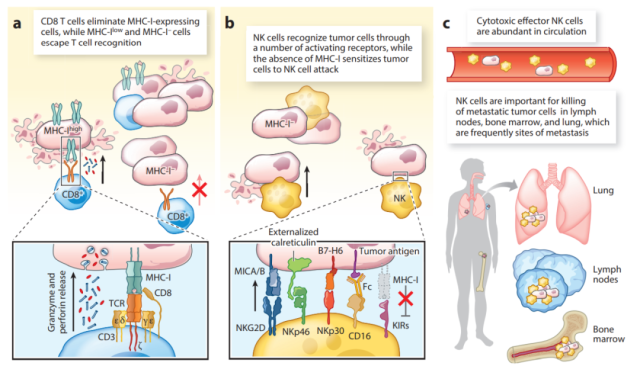
In contrast, NK cells utilize a series of activating and inhibitory receptors to recognize stressed and transformed cells, such as NKG2D, NKp46, NKp30 and NKp44 receptors.
The fact that NK cell recognition of tumor cells does not depend on any unique activating receptor compared to T cells provides greater flexibility in recognizing stressed and transformed cells.
Therefore, T cells and NK cells exert different selective pressures on tumor cells, providing a theoretical basis for the dual targeting of tumors by T cells and NK cells.
Shared receptors for T cells and NK cells
T cells and NK cells are often considered biologically as a strict distinction between adaptive immune recognition and innate immune recognition.
However, there is also substantial overlap in the receptor – ligand systems of NK cells and CD8+ T cells.
Thus, activating and inhibitory receptors expressed by CD8+ T cells and NK cells provide an opportunity to engage them in synergistic antitumor immunity.
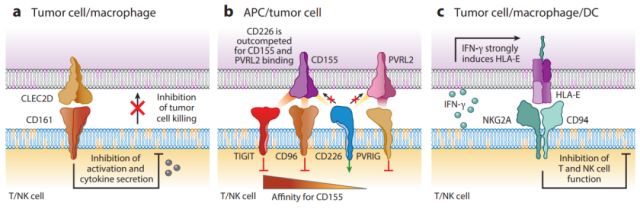
CD161 and its ligand CLEC2D
The CD161 receptor belongs to the C-type lectin receptor family and forms homodimers.
It was originally identified as an inhibitory receptor on NK cells that blocks killing of tumor cells expressing CLEC2D ligands.
Recently, CD161 was also identified as an important inhibitory receptor of tumor-infiltrating T cells.
CLEC2D is a C-type lectin receptor expressed on tumor cells and infiltrating myeloid cells of several human cancers.
CLEC2D is highly expressed in germinal center B cells.
Thus, B-cell lymphomas originating from germinal center B cells can express high levels of CLEC2D, including follicular lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma with germinal center B-cell-like gene expression Tumor subtype.
DCs and B cells also express CLEC2D following Toll-like receptor ( TLR ) activation. Therefore, targeting this inhibitory receptor can enhance the antitumor activity of T cells and NK cells.
NKG2A/CD94 and its ligand HLA-E
HLA-E is a nonclassical MHC Ib molecule that binds to the inhibitory NKG2A/CD94 receptor expressed by NK cells and CD8+ T cell subsets.
In many solid tumors, HLA-E is overexpressed on tumor cells, and expression of this protein has also been detected on tumor-infiltrating macrophages and DCs.
The NKG2A/CD94 receptor is constitutively expressed by most circulating and tumor-infiltrating NK cells.
Although only a small fraction of blood CD8+ T cells express NKG2A/CD94, its expression is upregulated in tumor-infiltrating CD8+ T cells that co-express PD-1 and other inhibitory receptors.
CD226 receptor and inhibitory counterpart receptors TIGIT, CD96 and PVRIG
CD226 receptor is an important co-stimulatory receptor expressed by NK cells and CD8+ T cells.
The primary ligand of this activating receptor, CD155, is widely expressed in human cancer cells, and in addition, CD226 also binds to the secondary ligand, PVRL2 ( CD112 ).
Interestingly, the activation of CD226 receptor activity was antagonized by three inhibitory receptors TIGIT, CD96 and PVRIG.
TIGIT and CD96 inhibitory receptors have higher affinity for the shared CD155 ligand than CD226 and can outperform CD226 in ligand binding.
Of the three inhibitory receptors, TIGIT has been the most extensively studied. TIGIT is expressed by NK cells, CD8+ T cells and regulatory T cells ( Tregs ).
TIGIT is highly expressed on tumor-infiltrating CD8+ T cells in different solid tumors and lymphomas.
TIGIT is also an important inhibitory receptor in NK cells and contributes to NK cell dysfunction in tumors.
Both PD-1 and TIGIT inhibit CD226, but through different mechanisms: PD-1 dephosphorylates Tyr-322 in the cytoplasmic domain of CD226, whereas TIGIT exceeds CD226 in CD155 binding.
CD96 and PVRIG receptors are less studied. Inactivation of the CD96 receptor in CD8+ T cells or NK cells enhances antitumor immunity in mouse models, but its role in human cells is unknown. PVRIG is also expressed by T cells and NK cells, and its main ligand is PVRL2.
NKG2D receptors and their stress-inducible ligands
NKG2D receptors are expressed by human NK cells, CD8+ T cells, and innate T cells ( NKT cells and γδ T cells ).
Upon activation, NKG2D signals through the adapter protein DAP10, inducing perforin-dependent cytolysis of NK cells, γδT cells, and NKT cells; it also provides co-stimulatory signals to CD8+ T cells.
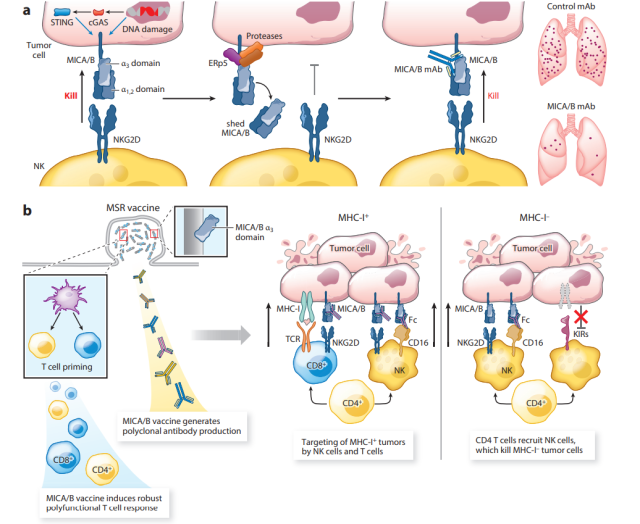
NKG2D receptors recognize a panel of ligands that are upregulated in stressed and transformed cells.
In humans, these ligands include MICA/MICB and ULBP1-6 proteins; in mice, these ligands include Rae1α–ε, H60a–c, and Mult1.
This receptor-ligand system is important for tumor immunity because these ligands are upregulated by DNA damage and cGAS STING signaling but are rarely expressed by healthy cells.
Essentially, upregulation of NKG2D ligands marks the clearance of stressed cells by cytotoxic lymphocytes.
Expression of NKG2D ligands is frequently detected in a variety of solid and hematological tumors, including prostate, ovarian, and breast cancers, as well as melanoma and multiple myeloma.
Proteolytic shedding of MICA/B leads to immune escape by dramatically reducing the density of these stimulatory NKG2D ligands on the tumor cell surface.
The disulfide isomerase ERp5 is involved in the initiation of shedding: it breaks structural disulfide bonds in the MICA/Bα3 domain, making it proteolytically accessible by ADAM-10/17 and MMP14.
Shedding MICA is strongly associated with disease progression in many human cancers but was not detected in the sera of healthy subjects.
NK cell-DC cell-T cell axis
NK cells not only act as cytotoxic effector cells, but also recruit DC cells into solid tumors, thereby forming T cell-mediated tumor immunity.
Traditional DCs are currently divided into two major subsets, cDC1 and cDC2 .
The cDC1 subset is critical for protective antitumor immunity by presenting cell-associated antigens of apoptotic tumor cells to CD8+ and CD4+ T cells .
In addition to this delivery role, cDC1 also plays a key role in tumors by recruiting and activating tumor-specific CD8+ T cells.
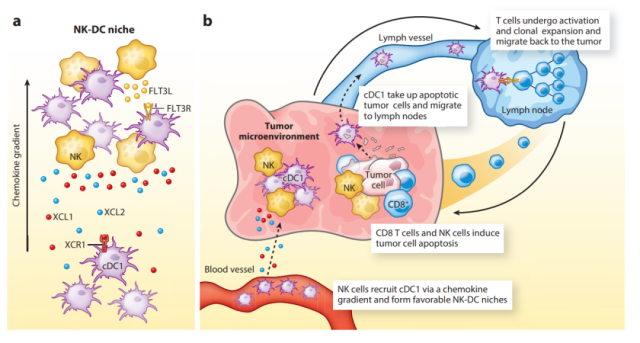
Two major studies have shown that NK cells play a key role in the recruitment of cDC1 to tumors.
The first study showed that NK cells secrete chemokines that mediate the recruitment of cDC1 to tumors, specifically XCL1, XCL2, and CCL5.
This is an important finding because cDC1 selectively expresses the XCR1 chemokine receptor of XCL1/2.
The second study focused on FLT3 ligand, a key cytokine that regulates DC differentiation and survival.
The expression of this important cytokine was detected in NK cells. The study showed that depletion of NK cells ( but not T cells ) greatly reduced the recruitment of cDC1 to tumors.
These studies demonstrate an important link between NK cell and T cell responses in tumors: NK cells recruit and support the survival of DCs, which in turn are critical for T cell-mediated tumor immunity.
Immunotherapy approach targeting synergy between T cells and NK cells
Cancer vaccine
Most current cancer vaccines focus on peptide epitopes, requiring individualized design due to the enormous diversity of MHC alleles among individuals.
In addition, cytotoxic T cells exert enormous selective pressure, leading to the emergence of tumor clones with downregulated or absent MHC-I expression.
A vaccine that elicits a dual attack by T cells and NK cells prevents the emergence of MHC-I-deficient tumor clones because the loss of MHC-I expression makes tumor cells more susceptible to NK cell killing.
Human tumors evade recognition of activating NKG2D receptors by proteolytic shedding of activated MICA and MICB ligands on tumor cells.
Cancer vaccines targeting the MICA/Bα3 domain involved in proteolytic shedding induce high-titer antibodies that inhibit shedding and increase the density of MICA/B proteins on the tumor cell surface. The vaccine induced significant homing of multiple T cell and NK cell populations to tumors in mice.
This vaccine-induced antibody plays an important role in the anti-tumor immune response: tumor-bound MICA/B antibodies enhance tumor antigen cross-presentation by DCs to CD8+ T cells and activate CD16-Fc receptors on NK cells. Increased NK cell-mediated tumor cell killing.
Targeting inhibitory cytokines
TGF-β is a major immunosuppressive cytokine that effectively suppresses T-cell and NK-cell functions within tumors.
TGF-β strongly inhibits T cell proliferation and effector function. In NK cells,
TGF-β signaling inhibits key metabolic programs regulated by mTOR, thereby suppressing proliferation and cytotoxicity.
TGF-β also inhibits the expression of multiple activating receptors on tumor-infiltrating NK cells, including the NKG2D receptor.
PGE2 plays an important role in immunosuppression and has also been linked to enhanced cancer cell survival and invasiveness.
Cyclooxygenase (COX)-1 and 2 are key enzymes for PGE2 synthesis and are frequently overexpressed in many human cancers.
Inhibition of PGE2 synthesis in mouse melanoma cell lines by inactivation of Ptgs1 and Ptgs2 genes resulted in TME shift from pro-tumor cytokines ( IL-1β and IL-6 ) to anti-tumor cytokines/chemokines ( IL-12 , IFN-γ and CXCL10 ) changes.
Importantly, CD103+cDC1 was significantly increased in the TME of these Ptgs1/2 − / − mice, and NK cells played a central role in the recruitment of cDC1 by secreting XCL1 and CCL5.
Engineered stimulatory cytokines
Several major cytokines act on T cells and NK cells simultaneously, including IL-2, IL-12, IL-15, and IL-18, providing an opportunity to enhance T cell and NK cell function.
For example, NKTR-214, a pegylated form of IL-2, in a phase 1 trial of NKTR-214 monotherapy resulted in stable disease in 14 of 26 patients (53.8%) with circulating CD4 +T cells, CD8+T cells and NK cells proliferated in large numbers.
Meanwhile, IL-15 is also being vigorously developed as a therapeutic drug because it activates effector T cells and NK cells, but not Treg.
IL-15 is important for the survival of memory T cells and induces NK cell proliferation and activation.
In a phase 1 clinical trial of patients with relapsed hematologic malignancies, the IL-15 superagonist ALT-803 induced treatment responses in 19% of treated patients.
Consistent with its mechanism of action, ALT-803 induced significant proliferation of NK cells and CD8+ T cells in blood, and enhanced expression of activating receptors ( NKG2D, NKp30 ) and granzyme B by circulating NK cells.
Combination therapy combining T cells and NK cells
A combination of four drugs, AIPV for short, consisting of an antitumor antibody (A), half-life-extended IL-2 (I), anti – PD -1 ( P ) and peptide vaccine ( V ).
This regimen can be further simplified to a single dose of AIP (anti-tumor antibody, IL-2 and PD-1 antibody).
Single-dose AIP treatment induced a rapid early response with significant effects on NK cells and macrophages, including upregulation of pro-inflammatory chemokines and cytokines.
Tumor-binding antibodies and half-life-prolonged IL-2 contribute to early NK cell activation, which enables the recruitment of DCs and CD8+ T cells, thereby rendering tumors more sensitive to ICB.
In addition, innate immune stimulators also provide opportunities to activate CD8+ T cells and NK cells. STING agonists induce robust antitumor responses mediated by CD8+ T cells and NK cells in mouse models.
STING agonists showed strong synergy with IL-2 by mobilizing T cells and NK cells against tumors in MHC-I-deficient and MHC-I-positive mice.
Summary
Recent studies have shown that the biology of T cells in tumors is highly correlated with that of NK cells.
NK cells play an important role in T cell-mediated tumor immunity by recruiting DC cells and supporting their survival.
Although NK cells and T cells belong to the innate and adaptive branches of the immune system, they also share an important system of activating and inhibitory receptor ligands, providing opportunities for immunotherapy.
Future studies should focus on investigating the environmental niches formed by DCs, NK cells, and T cells in mouse and human tumors, and identifying the molecular mechanisms that inhibit NK and T cell recruitment and their function in tumors.
A deeper molecular understanding of these features of human tumors may uncover novel immunotherapy targets and guide the development of combination therapies that effectively engage DCs, NK cells, and T cells.
references:
1. Designing Cancer Immunotherapies That Engage T Cells and NK Cells. Annu Rev Immunol. 2022 Nov 29.
How to play a synergistic role between T cells and NK cells?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



