Advantages and Challenges of CAR-T CAR-NK and CAR-M in cancer treatment
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Advantages and Challenges of CAR-T CAR-NK and CAR-M in cancer treatment
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Advantages and Challenges of CAR-T CAR-NK and CAR-M in cancer treatment.

Advantages and Challenges of CAR-T CAR-NK and CAR-M in cancer treatment
However, CAR-T cells still have some shortcomings, such as side effects, toxicity, T cell exhaustion, and show very low efficacy in the treatment of solid tumors.
At present, CAR-NK, CAR-NKT, CAR-macrophage (CAR-M), CAR-Treg, CAR-γδT and other new cell therapies with CAR technology as the core have sprung up, especially CAR-NK and CAR-M It shows great promise in the immunotherapy of tumors.
A Brief History of CAR-T Cell Development
Looking back at the history of CAR-T development, we must first mention the first bone marrow transplantation of leukemia patients reported by Thomas and his colleagues in 1957 and the subsequent discovery of the origin of T cells by Miller et al.
However, it wasn’t until Steven Rosenberg reported a study on tumor-infiltrating lymphocytes ( TIL ) in 1986 that people focused on the idea that a patient’s own immune cells could fight their own cancer.
In 1992, Sadelain et al. successfully established a method for retrovirus-mediated gene transfer to T lymphocytes, making genetic modification a means of controlling immunity in experimental or therapeutic settings.
Almost simultaneously, Zelig Eshhar and colleagues developed the first specific activation of cytotoxic lymphocytes by chimeric single-chain engineering using antibody binding domains and gamma or zeta subunits of immunoglobulins on T cell receptors. Generation of CAR-T cells.
Five years later, Dr. Sadelain’s research group demonstrated that integrating co-stimulatory signals such as CD28 into CAR-T can enhance survival, proliferation and maintain activity, thus developing the second generation of CAR.
Subsequently, CD19-targeted CAR-T cells were developed, and phase I clinical trials in chronic lymphocytic leukemia ( CLL ) and acute lymphoblastic leukemia ( ALL ) were initiated .
The results of the trial demonstrated that CAR-T therapy induced effective remission in adults with chemotherapy-refractory ALL, and the subsequent scale-up of bioprocess production.
In 2017, the FDA approved CD19 CAR-T cell therapy ( Tisagenlecleucel ) for ALL in children and young adults. So far, the FDA has approved five CAR-T cell therapy drugs for cancer treatment.

Challenges of CAR-T Cell Therapy
The challenges faced by CAR-T cell therapy are mainly related to side effects, toxicity, T cell exhaustion and malignant tumor microenvironment ( TME ).
In addition, the manufacturing process in large-scale production is currently time-consuming and expensive, making it an even greater challenge to get as many patients as possible to receive CAR-T cell immunotherapy.

Side Effects and Toxicity
This type of immunotherapy can have potentially fatal toxicity following CAR-T cell infusion. There are two main categories of toxicity: cytokine release syndrome ( CRS ) and neurotoxicity ( NTX ) or CAR-T cell-associated encephalopathy syndrome ( CRES ).
CRS or “cytokine storm” is a systemic inflammatory response caused by massive activation of lymphocytes ( B cells, T cells, and natural killer cells ) and myeloid cells ( macrophages, dendritic cells, and monocytes ) Clinical symptoms include fever, fatigue, headache, rash, arthralgia, and myalgia.
NTX is another common complication of CAR-T cell immunotherapy, occurring in more than 40% of patients. Patients exhibit symptoms such as confusion, slowness, tremors, delirium, difficulty finding words, and headaches .
CAR-T cell exhaustion
Although the complete remission rate of CAR-T cell therapy is high, most patients who achieve remission show disease relapse within a few years, and the relapse rate of B-ALL ranges from 21% to 45%, and increases with the extension of follow-up time . Treatment failure is partly due to the depletion of CAR-T cells by the TME generated by solid tumors.
CAR-T cell exhaustion refers to a dysfunctional state characterized by the loss of antigen-specific T cells due to persistent antigen stimulation, increased expression of costimulatory domains of CAR structures, and inhibitory receptors.
In vitro CAR-T cell studies have shown that the expression of inhibitory receptors ( such as PD-1, Lag3, Tim3, and TIGIT ) is upregulated during CAR-T cell exhaustion, and the inhibition of the PI3K/AKT pathway through CTLA-4 is the cause of The main reason for the loss of anti-tumor function.
Cytokines also play an important role in this, as exhausted CAR-T cells reduce the ability to express and secrete IL-2, TNF-α, and IFN-γ. Other factors, such as transcription factors, metabolism, and epigenetic modifications, also play important roles in the development of CAR-T cell exhaustion.
Tumor microenvironment
CAR T cell immunotherapy has not been successful in solid tumors. One possible reason is that the immunosuppressive nature of TME affects the efficacy of adoptive immunotherapy. Solid tumors present highly infiltrating stromal cells, such as cancer-associated fibroblasts ( CAFs ), and suppressive immune cells, including myeloid-derived suppressor cells ( MDSCs ), tumor-associated macrophages ( TAMs ), tumor-associated neutrophils ( TAN ), mast cells, and regulatory T cells ( Treg ), which contribute to the establishment of an immunosuppressive TME that can interfere with the efficacy of CAR-T cell therapy.
Research overview of CAR-NK
NK cells, the first identified subtype of innate lymphoid cells ( ILCs ), can exert multiple effector functions on virus-infected and/or transformed cells, primarily cell killing and production of pro-inflammatory cytokines.
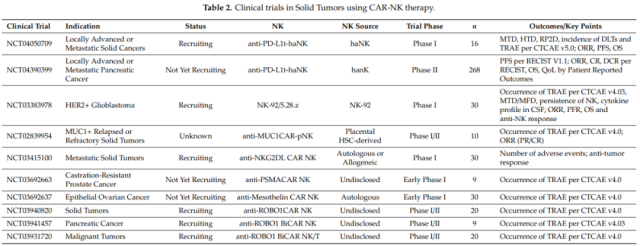
The research on CAR-NK therapy is currently in its infancy, and the number of preclinical and clinical studies is increasing year by year , which is reflected in the increasing number of research papers on CAR-NK every year.

Furthermore, in terms of targets studied, Her2 is the most commonly used target in solid tumors, while the CD19 antigen is most common in hematological tumors.
Of the studies using primary NK cells, 65% were studying B-cell malignancies, with CD19 being the most popular target. Interestingly, in studies using NK cell lines, more than twice as many solid tumors were studied as hematologic malignancies.

In terms of clinical research, CD19-CAR-NK cells have a high response rate to hematological tumors. In addition to CD19, clinical studies of CAR-NK cells in lymphoma and leukemia also target CD7 ( NCT02742727 ) and CD33 ( NCT02944162 ). Currently, several clinical trials of CAR-NK cells targeting hematological malignancies are underway.

There are also multiple studies in solid tumors that are in the initiation or recruiting stages.
Advantages and challenges of CAR-NK
Like CAR-T cells, CAR-NK cells have extracellular, transmembrane and intracellular signaling domains.
NK cells increase their cytotoxic capacity and cytokine production through two other co-stimulatory molecules, NKG2D and CD244 ( 2B4 ).
Therefore, it has stronger tumor-specific targeting and cytotoxicity than CAR-T cells.
CAR-NK cell therapy may become an alternative to CAR-T therapy in the future, because CAR-NK cells have several unique features beyond CAR-T as follows.

First, allogeneic NK cells are fairly safe for adoptive cell therapy ( ACT ) because they generally do not mediate GVHD. In addition, NK cells secrete only small amounts of IFN-γ and GM-CSF, and do not produce IL-1 and IL-6 that initiate CRS.
Second, in addition to inhibiting cancer cells by recognizing tumor surface antigens through single-chain antibodies, NK cells can also inhibit cancer cells by recognizing various ligands through multiple receptors, such as natural cytotoxicity receptors (NKp46, NKp44, and NKp30 ) , NKG2D and DNAM-1 ( CD226 ).
Finally, NK cells are very abundant in clinical samples and can be generated from peripheral blood ( PB ), umbilical cord blood ( UCB ), human embryonic stem cells ( HESC ), induced pluripotent stem cells ( IPSC ) and even the NK-92 cell line.
At the same time, CAR-NK is also facing some challenges.
First, the lack of in vivo persistence of infused cells in the absence of cytokine support is one of the major drawbacks of adoptive NK cell therapy.
While it may be safer, it also limits the effectiveness of NK cell immunotherapy.
Second, rapid homing to the tumor bed is critical for adoptive cell therapy efficacy and is governed by complex interactions between NK cells and chemokines released by tumor cells. However, the efficiency of NK cell homing to tumor sites has been debated, thus prompting ongoing efforts to improve it.
Third, similar to CAR-T, TME includes immunosuppressive molecules, immunosuppressive cells and an unfavorable environment that hinders the function of immune cells, which is the main obstacle for CAR-NK cell therapy.
Finally, lentivirus-based transduction systems are one of the most commonly used methods for intracellular gene modification and delivery. However, NK cells are resistant to lentivirus due to their natural properties, which makes lentivirus-based transduction a challenge.
Research progress of CAR-M
In view of the success of CAR-T cell therapy and the development potential of CAR-NK cells, researchers have generated great interest in the development of CAR macrophages ( CAR-M ) for tumor immunotherapy.
The emergence of CAR-M has opened up new possibilities for the treatment of solid tumors: modifying human macrophages with specific CARs to improve the phagocytic activity and antigen presentation of macrophages to tumors.
There have been multiple attempts to use CAR-M to treat cancer. The researchers engineered chimeric antigen receptor phagocytes ( CAR-P ), which guide macrophages to engulf specific target cells.

Studies have shown that CAR-P expressing the intracellular domain of Megf10 or FcRv can promote the phagocytosis of target antigens.
CAR-PMegf10 can specifically trigger the phagocytosis of targeting ligands and initiate phagocytosis through a local signaling cascade of tyrosine phosphorylation, and TCR-CD3ζ may promote the phagocytosis of CAR-P by recruiting syk kinase.
Whole-cell phagocytosis was relatively rare, while biting target cells was more frequent, suggesting that the interaction between CAR-P macrophages and target cells was not sufficient to trigger direct whole-cell phagocytosis.
It has been found that PI3K signaling plays an important role in the endocytosis of large targets and can promote the phagocytosis of macrophages.
The researchers linked the pI3K p85 subunit to CAR-P-FcRv to form a “tandem” CAR ( CAR-Ptandem ). CAR-Ptandem has a good whole-cell phagocytosis function.

Researchers at the University of Pennsylvania used an anti-HER2 CAR-M containing the CD3-ζ intracellular domain.
In two heterotopic mouse models transplanted with solid tumors, a single injection of anti-HER2-CAR-M reduced tumor burden and prolonged mouse survival.
It was also found in a humanized mouse model that HER2-CAR-M can convert M2 macrophages into M1 macrophages, induce an inflammatory tumor microenvironment, and enhance the anti-tumor cytotoxicity of T cells.
In addition, it was also found that HER2-CAR-M may produce epitope diffusion, which provides a new idea for avoiding tumor immune escape.

Zhang et al. used induced pluripotent stem cells ( iPSCs ) to express CAR constructs and differentiate into macrophages, named CAR-iMac.
Studies have shown that CAR-expressing iPSCs can differentiate into macrophage-like cells, and in the absence of antigen, CAR-iMac is closer to the M2 polarization state.
However, in the presence of specific antigens such as CD19, CAR-mediated signaling promoted the phagocytosis of CAR-iMacs and resulted in the conversion of CAR-iMacs to the pro-inflammatory M1 phenotype.
iPSC-derived macrophages may become an important cell source for immunotherapy of myeloid tumors.

An important reason why CAR-T is ineffective in treating solid tumors is that it is difficult for T cells to enter tumor tissue.
This is because the physical barrier formed by the extracellular matrix ( ECM ) of solid tumors prevents T cells from entering tumor tissue.
ECM is produced by highly ordered fiber molecules, glycoproteins and other macromolecules, and its synthesis and degradation are mainly regulated by matrix metalloproteinases (MMPs ) and tissue inhibitors of metalloproteinases ( TIMPs ), and macrophages are an important source of MMPs .
The researchers designed a CAR-147 structure consisting of a single-chain antibody targeting human HER2, the hinge region of IgG1, and the transmembrane and intracellular regions of the mouse CD147 molecule.
After co-cultured with HER2+ human breast cancer cells, CAR-147 induced the expression of multiple MMPs in CAR-147 macrophages, proving that CAR-147 can specifically recognize HER2 antigen and effectively activate the expression of MMPs in macrophages.
CAR-147 macrophages did not inhibit tumor cell proliferation in vitro, but intravenous injection of CAR-147 macrophages significantly inhibited tumor growth in a mouse model of 4T1 breast cancer.
At the same time, it was found that the proportion of T cells in tumors treated with CAR-147 macrophages was much higher than that of tumors treated with control macrophages, indicating that CAR-147 macrophages can destroy the extracellular matrix of tumors and promote the infiltration of T cells into tumors .
Clinical application of CAR-M in the treatment of solid tumors
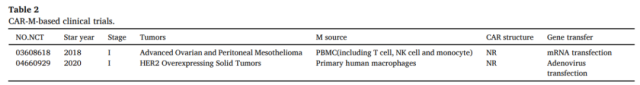
Until November 2020, two clinical trials based on the CAR-M strategy have been approved by the FDA.
The first is drug candidate CT-0508 from CARISMA Therapeutics, which treats patients with relapsed/refractory HER2-overexpressing tumors with an anti-HER2 CAR-M (Phase I clinical trial ) .
The other is Maxyte’s MCY-M11, which uses mRNA to transfect PBMCs to express mesothelin-targeted CARs ( including CAR-M ) for the treatment of patients with relapsed/refractory ovarian cancer and peritoneal mesothelioma, and is currently recruiting volunteers Conduct phase I clinical trials.
Regarding the clinical transformation of CAR-M, there are several aspects that need attention: First, the safety and effectiveness of CAR-M are the basis of this therapy.
Although it has been verified by animal experiments, the effect of CAR-M in humans Safety and efficacy still need to be verified;
secondly, reliable cell source and expansion are necessary conditions for the clinical application of CAR-M, and CAR-M can be prepared from PBMC or iPSCs.
Furthermore, unlike T cells, macrophages have a lower risk of developing GVHD, which means that products can be manufactured ahead of time for patients to use on demand.
Third, there is another problem that must be considered. At present, CAR-M mostly uses virus transfection methods, which may induce insertion mutations. CRISPR/Cas9 provides a new possibility to solve this problem, and it only takes one week to complete Editing of CAR-T genes.
Advantages and challenges of CAR-M
Similar to CAR-T and CAR-NK cells, CAR-M cells consist of an extracellular signaling domain that recognizes a specific tumor antigen, a transmembrane region, and an intracellular activation signal region.
Currently, studies on extracellular signaling domains are mainly focused on several common tumor targets, such as CD19 and HER2.
Unlike CAR-T cells, CAR-M cells have the following three advantages.
1) Due to the physical barrier formed by the stroma around tumor cells, T cells cannot enter the tumor environment, while macrophages can obviously infiltrate into the tumor environment. TAMs play important roles in tumor invasion, metastasis, immunosuppression and angiogenesis. CAR-M can reduce the ratio of TAM, affect the cell phenotype of TAM, and have a positive effect on the treatment of tumors.
2) In addition to the function of phagocytosis of tumor cells, CAR-M also has the function of promoting antigen presentation and enhancing T cell killing.
3) Compared with CAR-T, CAR-M has limited circulation time and less non-tumor targeting toxicity.
Although CAR-M has great potential to become a powerful tumor immunotherapy method, many problems need to be overcome to achieve the desired effect. The first is the limitation of the number of cells: macrophages do not proliferate either in vitro or after injection in vivo. Patients can only receive a limited number of macrophages, which may affect the efficacy of the treatment. The second is related to the migration characteristics of macrophages in vivo. After injection, exogenous macrophages pass through the lungs, and most of them stay in the liver, which is not conducive to the treatment of cancer. The third is the complex tumor microenvironment. Although CAR-M has achieved good results in mouse models, the actual tumor microenvironment in humans is much more complex than that in animal models. Finally, due to the high heterogeneity of tumor cells, the expression of the target antigen may be insufficient. This problem has been very prominent in CAR-T therapy. Clinical studies have found that most tumor cells removed by CAR-T cells have high levels of target antigen expression. It is foreseeable that this will also become a major obstacle to the development of CAR-M therapy.
Summary: Advantages and Challenges of CAR-T CAR-NK and CAR-M in cancer treatment
NK cells are a unique class of anti-tumor effector cells with functions such as cytotoxicity, cytokine production, and immune memory that are not restricted by MHC . CAR-NK cell therapy is a promising field of clinical research with good safety and preliminary efficacy in certain cancer patients.
CAR-M therapy has shown its effective anti-tumor ability in animal experiments. Compared with CAR-T and CAR-NK , CAR-M has its unique advantages as a new cellular immunotherapy, but there are also many shortcomings that need to be overcome.
Therefore, attention should be paid to maximizing the effectiveness and safety of CAR-M in future clinical treatment .
We have reason to believe that with the further development of science and technology, CAR-NK and CAR-M will soon show curative effect in patients, adding another boost to tumor immunotherapy!
Advantages and Challenges of CAR-T CAR-NK and CAR-M in cancer treatmentreferences:
1. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. JHematol Oncol. 2021; 14: 73.
2. Natural Born Killers: NK Cells in Cancer Therapy. Cancers (Basel). 2020 Jul 31;12(8):2131
3. CAR-macrophage: A newimmunotherapy candidate against solid tumors. Biomed Pharmacother. 2021 Jul;139:111605.
Advantages and Challenges of CAR-T CAR-NK and CAR-M in cancer treatment
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



