Results of Human Challenge Test Trials for Actively Infection with COVID-19
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Results of Human Challenge Test Trials for Actively Infection with COVID-19
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Lancet Microbe Publishes Results of Human Challenge Test Trials for Actively Infection with COVID-19.
Recruiting healthy individuals for voluntary COVID-19 infection with £4,500 per person, the world’s first human challenge trial for COVID-19 has made new progress.
On June 9, 2023, The Lancet Microbe published a paper titled “Viral emissions into the air and environment after SARS-CoV-2 human challenge: a phase 1, open-label, first-in-human study,” revealing the latest results of the human challenge trial for COVID-19.

Funding of £33.6 million and £4,500 per person
In February 2021, the Imperial College London School of Medicine, in collaboration with the UK Vaccine Taskforce and the National Institute for Health Research (NIHR), announced its collaboration with the Royal Free Hospital in London and the clinical trial design company hVIVO to conduct the “COVID-19 Human Challenge Trial” with the code name COVHIC001.

Source:UKcovidchallenge
For this trial, the UK government provided special funding of £33.6 million to support the research. According to a 2021 report by London’s Evening Standard, the research team paid each volunteer a reward of £4,500.
The so-called human challenge trial involves selecting volunteers who have not been infected with the COVID-19 virus and artificially “infecting” them with the virus under controlled conditions to observe the changes in the human body after infection.
As early as 2020, the World Health Organization (WHO) issued specific guidelines for human challenge trials of COVID-19 vaccines, stating that well-designed challenge trials can not only accelerate the development of COVID-19 vaccines but also potentially make the widely administered vaccines more effective.
However, there are also voices that argue that human challenge trials expose volunteers to avoidable risks and may contradict medical ethics.
Amidst ongoing controversies, the study successfully enrolled volunteers in July 2021. In March 2022, the research team published their findings in Nature Medicine for the first time.

A total of 34 healthy young individuals aged 18 to 29, who had neither contracted COVID-19 nor received the vaccine, were enrolled. After “inoculation” with a fixed dose of the COVID-19 virus, 18 individuals became infected. The researchers observed the following:
- 14 days after inoculation, some subjects still tested positive for viral nucleic acid. At 28 days, 33% and 11% of individuals still tested positive for viral nucleic acid in their throat and nasopharynx, respectively. However, infected individuals were no longer infectious an average of 10.2 days after virus exposure.
- Neutralizing antibodies in the blood of infected individuals peaked 28 days after infection.
- 89% of virus-infected individuals exhibited symptoms, with the onset of symptoms occurring between 2 and 4 days after detection of viral infection. Twelve infected individuals experienced a degree of olfactory impairment. Even after six months, five individuals reported incomplete recovery of their sense of smell.
Latest research results and recruitment for six rounds
After obtaining the above results, the human challenge trial did not end. In the latest published paper, researchers answered: How does the coronavirus spread from patients to the air? Which type of patients are most likely to transmit the virus?
This research is also based on data from COVHIC001, with the participation of 18 volunteers who were infected with COVID-19 in the environmental transmission test.
After confirming that the volunteers were in the recovery period, the researchers collected nasal and throat swab samples from the volunteers every day. The volunteers were required to wear sampling masks for 60 minutes each morning and air samples were collected 1 meter away from the volunteers’ heads using air samplers.
In addition, the researchers also collected environmental swabs from the volunteers’ hands and hand-contact surfaces (bed, TV remote control, sink, toilet, etc.), and the volunteers were required to complete a symptom diary three times a day.
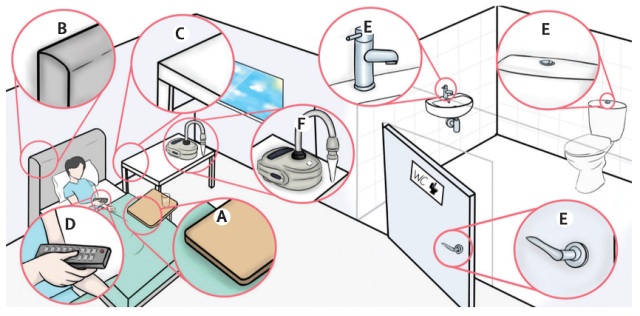
After all 18 volunteers had recovered from the infection, the researchers analyzed the data and found the following results:
- The virus was detectable in the room air and/or environmental samples of all 18 volunteers.
- In the room air samples taken from 16 volunteers within 2-14 days after viral inoculation, 25% showed detectable SARS-CoV-2 RNA. In the masks worn by 17 volunteers within 2-14 days after viral inoculation, 43% showed detectable SARS-CoV-2 RNA. In the environmental swab samples taken from all volunteers within 2-14 days after viral inoculation, 29% showed detectable SARS-CoV-2 RNA, with the highest virus-positive rate in swabs collected from the bathroom (34.5%).
- The virus was culturable in the masks worn by volunteers and some environmental swabs, but not in the hand swabs.
- The analysis of the viral load in swabs from different parts of the body and environmental samples (including environmental swabs and air samples) showed a strong correlation between the viral load on facilities manipulated by hand (TV remote control, bathroom door handle) and the viral load on hand swabs. The viral load in masks, hand swabs, and environmental samples showed a stronger correlation with the viral load in nasal swabs than with the viral load in throat swabs.
- The severity of infection symptoms and the level of viral shedding were almost unrelated. In other words, volunteers with the most severe symptoms did not necessarily shed more virus than those with milder symptoms, and in some cases, they may even shed less.
Based on these findings, the researchers believe that the virus primarily spreads from the nasal mucosa of COVID-19 infected individuals, especially the highly susceptible epithelial cells in the mucosa.
Therefore, using nasal swab specimens to assess the infectivity of COVID-19 patients is more appropriate than using throat swab specimens.
For the general population, this result highlights the importance of fully covering the nose when wearing masks.
Furthermore, the researchers also believe that based on the correlation between viral shedding patterns, peak shedding, and symptoms among volunteers, promoting awareness of early symptoms of COVID-19 infection and the use of self-test antigen kits at the community level can help reduce community transmission of the virus.
Based on the positive results obtained from the COVHIC001 study, researchers at Imperial College London have started the second round of the “COVID-19 Human Challenge,” namely the COVHIC002 study.
According to information on the official website [3], the second round of the study will mainly investigate the biological responses after human infection with the coronavirus, including the protective effects of initial infection against secondary infection and factors influencing susceptibility and/or severity of COVID-19 infection.
Multiple vaccines will also be tested for their preventive effects against COVID-
Reference:
[1]Killingley B, Mann AJ, Kalinova M, et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. 2022;28(5):1031-1041. doi: 10.1038/s41591-022-01780-9
[2]Zhou J, Singanayagam A, Goonawardane N, et al. Viral emissions into the air and environment after SARS-CoV-2 human challenge: a phase 1, open label, first-in-human study. Lancet Microbe. 2023:S2666-5247(23)00101-5. doi: 10.1016/S2666-5247(23)00101-5
[3]Imperial College London. COVID-19 Human Challenge Study: COVHIC002 —— About this study. https://www.imperial.ac.uk/infectious-disease/research/human-challenge/covhic002/about-this-study/
[4]Imperial College London. COVID-19 Human Challenge Study: COVHIC002. https://www.imperial.ac.uk/infectious-disease/research/human-challenge/covhic002/
Results of Human Challenge Test Trials for Actively Infection with COVID-19
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



