Nearly 6.4 Million New Cases: Searching for the Next Tuberculosis Vaccine
- EB Virus Could Be Infected by Kiss: A Hidden Threat Linked to Cancer
- The Silent Threat: How Gas Stoves Pollute Our Homes and Impact Health
- Paternal Microbiome Perturbations Impact Offspring Fitness
- New Report Casts Doubt on Maradona’s Cause of Death and Rocks Manslaughter Case
- Chinese academician unable to provide the exact source of liver transplants
- Early Biomarker for Multiple Sclerosis Development Identified Years in Advance
Nearly 6.4 Million New Cases: Searching for the Next Tuberculosis Vaccine
- AstraZeneca Admits for the First Time that its COVID Vaccine Has Blood Clot Side Effects
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Nearly 6.4 Million New Cases: Searching for the Next Tuberculosis Vaccine.
Since the first administration of the BCG vaccine in 1921, there hasn’t been another approved vaccine for the treatment of tuberculosis (TB).
However, due to persistently high death rates associated with tuberculosis, the global demand for a new TB vaccine is steadily increasing.
Tuberculosis, caused by Mycobacterium tuberculosis, spreads when infected individuals release the bacteria into the air (e.g., through coughing). Despite a reduction in TB cases during the COVID-19 pandemic, the incidence is again on the rise, with nearly 6.4 million new cases reported globally, according to the World Health Organization (WHO). Researchers anticipate that TB incidence will soon revert to levels seen before 2020, making it the deadliest global infectious disease.
The BCG vaccine, a live attenuated vaccine, has also been explored for cancer treatment, COVID-19 prevention, and even as a potential Alzheimer’s disease therapy. However, its effectiveness is limited in individuals with existing virus antibodies in the bloodstream, excluding a significant portion of the population. Moreover, its efficacy is only around 22%. While the BCG vaccine has weak preventive effects against TB in adults, TB is primarily spread by adults.
Despite the lack of options, researchers remain cautiously optimistic about the new vaccines in development. Dr. Helen McShane, a vaccine specialist at the University of Oxford, stated, “Twenty-three years ago, there were no new TB vaccines in clinical trials, and now we have 12 different vaccines in various stages of development being studied clinically.”
New Developments in Tuberculosis
In a prior Phase I clinical trial (NCT03722472), researchers have been able to test the first heat-stable TB vaccine in patients. In this trial, patients received either a heat-stable version or a non-heat-stable version of the vaccine. Results showed that in both groups, participants’ antibody responses remained elevated within six months after the final vaccine dose.
The Infectious Disease Research Institute (IDRI) is developing an ID93 vaccine. The trial examined a heat-stable formulation of the previously tested ID93 + GLA-SE adjuvant subunit vaccine, in collaboration with the National Institutes of Health (NIH). The trial results indicated that participants maintained elevated antibody responses within six months after receiving the heat-stable and non-heat-stable vaccine formulations.
According to Dr. Fox, the lead scientist of the trial, this is a significant advancement in TB vaccine research as the current BCG vaccine requires refrigeration. He explained that this new vaccine can be stored at 37°C for up to three months, making storage viable in rural areas with high TB incidence.
Compared to live attenuated vaccines, adjuvanted protein subunit vaccines like ID93 are considered safer with lower risks of side effects. Adjuvants enhance antigen-specific immune responses. “A few candidate vaccines are now taking this approach, and have entered Phase II clinical trials for efficacy testing,” mentioned NIH, which is sponsoring a Phase II trial of the ID93 + GLA-SE adjuvant subunit vaccine in South Africa. Additionally, the Statens Serum Institute in Copenhagen, Denmark, is developing a subunit adjuvant vaccine named SSIH-56IC31 for TB prevention.
McShane predicts that the new vaccine pipelines could also address drug-resistant TB strains. “We expect that all the vaccines in development will work as well against drug-resistant strains as they do against drug-sensitive ones.”
The Bill and Melinda Gates Medical Research Institute (MRI) is sponsoring a large Phase III trial with 26,000 participants. This trial investigates a protein adjuvant vaccine, M72/AS01E, composed of M72 recombinant fusion protein and the AS01 adjuvant. Originally developed by GSK, the project was abandoned by the company after a successful Phase IIb study.
On June 28th, the Bill and Melinda Gates Foundation and the Wellcome Trust announced a $550 million funding to advance the Phase III clinical trial of the M72/AS01E TB vaccine. The Gates Foundation stated that if successful, this Phase III trial could mark the first new TB vaccine to enter the market in over a century.
In terms of efficacy, Phase 2b clinical trial results showed that participants receiving M72/AS01E had 10 cases that met the case definition for TB infection, compared to 22 cases in the placebo group, resulting in a vaccine efficacy of 54%.
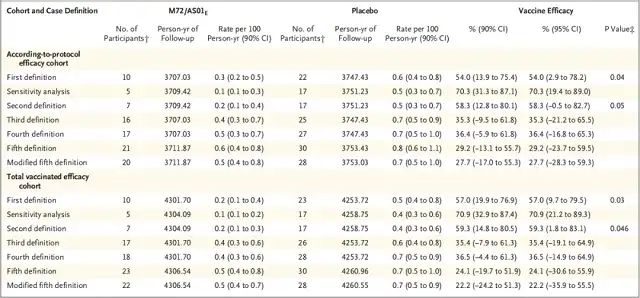
Figure: Comparison of efficacy of M72/AS01 and placebo (Source: Phase 2b Controlled Trial of M72/AS01E Vaccine to Prevent Tuberculosis.)
According to GlobalData, there are currently six TB vaccines in Phase III clinical trials. The Gamaleya Federal Center of Epidemiology and Microbiology is developing a protein subunit vaccine called GamTBvac. GC Biopharma is conducting Phase III trials for its live attenuated vaccine, GC-3107A, to replace the BCG vaccine. Additionally, the Serum Institute of India is developing its own live attenuated vaccine as an alternative to infant BCG vaccination and for use in adults.
Tuberculosis Advances
In a prior Phase I clinical trial (NCT03722472), researchers have tested the first heat-resistant TB vaccine on patients.
The trial involved administering both heat-stable and non-heat-stable versions of the vaccine, showing that antibody responses remained elevated within six months of the final vaccine dose.
The Infectious Disease Research Institute (IDRI) is developing an ID93 vaccine.
The trial investigated a heat-stable formulation of the previously tested ID93 + GLA-SEDr adjuvant subunit vaccine, developed in collaboration with the U.S. National Institutes of Health (NIH). Results indicated that participants’ antibody responses continued to increase for six months after receiving the heat-stable and non-heat-stable vaccine formulations.
Dr. Fox, the lead scientist of the trial, explained that this new vaccine could be stored at 37°C for up to three months, a critical development since the current BCG vaccine requires refrigeration, enabling storage in rural areas with higher TB incidence.
Compared to live attenuated vaccines, adjuvant protein subunit vaccines pose fewer risks and can enhance antigen-specific immune responses. “A small number of candidate vaccines are now using this approach and have entered Phase II efficacy testing,” said NIH, which sponsors a Phase II trial of the ID93 + GLA-SEDr adjuvant subunit vaccine in South Africa. Additionally, the Statens Serum Institute in Denmark is developing an adjuvant subunit vaccine named SSIH-56IC31 for TB prevention.
McShane anticipates that the new vaccine pipeline could also address drug-resistant TB. “We anticipate that all the vaccines in development will work against drug-resistant and drug-sensitive strains.”
The Bill & Melinda Gates Medical Research Institute (MRI) is sponsoring a widespread Phase III trial involving 26,000 participants.
This trial investigates the protein adjuvant vaccine M72/AS01E, composed of M72 recombinant fusion protein and AS01 adjuvant.
Although initially developed by GSK, the project was abandoned by the company after a successful Phase IIb study.
On June 28, the Bill & Melinda Gates Foundation and Wellcome Trust announced $550 million in funding to drive the Phase III clinical trial of the M72/AS01E TB candidate vaccine.
If successful, this trial could mark the first new TB vaccine to be introduced in over a century.
As of now, six TB vaccines are in Phase III clinical trials, according to data from GlobalData. These include a protein subunit vaccine called GamTBvac by the Gamaleya Federal Center of Epidemiology and Microbiology, as well as GC Biopharma’s live attenuated vaccine GC-3107A, which aims to replace BCG. Additionally, the Serum Institute of India is developing its own live attenuated vaccine, suitable for infants and adults.
Stagnant Tuberculosis Research
The development of tuberculosis vaccines has remained stagnant due to the complexity of the virus, lack of research interest, and funding shortages, a situation that persisted until about 20 years ago.
“Unlike most viruses, which typically have 10 or 15 genes, the tuberculosis bacillus has around 3500 genes, and we don’t understand its virulence factors. We also don’t comprehend why out of 20 people infected with the tuberculosis bacillus, only one person falls ill – this is a huge question,” stated Schmidt. Dr. Keertan Dheda, a professor of Tuberculosis and Global Health at the University of Cape Town, also mentioned, “We don’t know which biomarkers can identify successful immunity to tuberculosis… This has been one of the challenges in tuberculosis vaccine development for decades, and currently, we still don’t have a good solution.”
The complexity of the virus and these unknown factors might pose challenges to clinical trials as they require larger patient cohorts.
McShane explained that efficacy trials for tuberculosis vaccines involve 1000 to 10,000 participants, costing tens of millions of dollars and possibly taking more than 5 years to complete. She added that with limited funding, researchers must carefully select which candidate drugs are worthy of the “massive undertaking” required for such studies.
Dheda mentioned, “One factor hindering research is funding.” Schmidt also added, “High-income countries don’t have a market, and there’s no significant disease burden… Usually, policymakers provide funding for diseases that they themselves need treatment for.” Clinical trials for tuberculosis are primarily funded through charitable means, but McShane noted that funding in this field has improved significantly in recent years, leading to some progress in tuberculosis vaccine development.
Poverty and overcrowding are key factors driving the spread of tuberculosis, and these remain challenging issues to address globally. “If we genuinely want to improve tuberculosis, the only hope is to have a good vaccine that can control it because we do have better diagnostics and drugs, but they haven’t made an impact,” said Dheda.
Source:
[1] Pharma Fix: The quest for the next tuberculosis vaccine. Pharmaceutical Technology. By Akosua Mireku. March 24, 2023.
[2] Efficacy of 54%! With no new vaccine in 102 years, will the world’s first be born?
Nearly 6.4 Million New Cases: Searching for the Next Tuberculosis Vaccine
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



