CDC Issues Updated Recommendation for XBB.1.5 COVID-19 Vaccine
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
CDC Issues Updated Recommendation for XBB.1.5 COVID-19 Vaccine
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
CDC Issues Updated Recommendation for XBB.1.5 COVID-19 Vaccine in the United States, Beneficial for Everyone
On September 12th, the CDC officially updated its recommendations for COVID-19 vaccination in the United States, advising that every individual aged six months and older should receive the updated version of the COVID-19 vaccine with XBB.1.5 as the antigen during the fall and winter of 2023.
Currently, two vaccines have been approved: the mRNA vaccines developed by Pfizer/BioNTech and Moderna. On September 11th, the FDA had just approved these two updated vaccines. However, unlike typical medications, vaccines not only require FDA approval for efficacy and safety but also necessitate recommendations from the CDC from a public health perspective, determining who should receive them and under what circumstances.
In this case, the efficacy and safety data for the two updated mRNA vaccines had already been confirmed during the FDA review process. In fact, at the FDA external expert committee meeting held in June of this year, both mRNA vaccine manufacturers (Pfizer and BioNTech as a collaboration counted as one) and the recombinant protein vaccine manufacturer Novavax provided updates on the next-generation vaccines.
During that meeting, several manufacturers and FDA staff also demonstrated why vaccine updates were necessary and why transitioning to a monovalent vaccine was a decision made. In short, the COVID-19 virus has been continuously evolving, and by 2023, the main virus strain had become XBB.1.5, which had distinct antigenic properties compared to the BA.4/5 vaccines used at that time. While the original two-dose BA.4/5 vaccine still reduced the risk of severe illness, XBB.1.5’s relative immune escape meant lower effectiveness in preventing infections and a faster decline in effectiveness against severe illness compared to BA.4/5. Due to the continuous evolution of the virus, regular vaccine updates to align with the predominant virus strain became necessary, similar to the approach for the flu.
The decision to update to XBB.1.5 had already been made, as well as the decision to transition to a monovalent vaccine. There were multiple considerations for this transition. The first update of COVID-19 vaccines, which occurred in the fall of 2022, included BA.1 or BA.4/5, both of which were bivalent, meaning half of the vaccine targeted Omicron (BA.4/5 in the United States, BA.1 in other countries), while the other half continued to use the original strain from when the vaccines were first launched.
The decision to retain the original strain was primarily due to its continued effectiveness and ability to provide protection against Omicron, making it a prudent choice for the initial vaccine update. Additionally, updating the vaccine aimed to broaden the immune recognition of individuals, but the significant differences between the original strain and Omicron made retaining the original strain important to prevent unpredicted changes in the virus and maintain the reliability of monovalent Omicron vaccines.
However, now that the original strain and non-Omicron viral strains have become rare, the significance of retaining the original strain’s antigen has diminished. Furthermore, the human immune system exhibits an immunological imprint, meaning repeated vaccinations with the original strain vaccine lead to habitual immune responses against the original strain, resulting in high levels of neutralizing antibodies against the original strain. However, this could have negative consequences for the desired broad-spectrum immunity, as it may reduce the production of immune responses against new antigens.
In this context, transitioning to a monovalent vaccine was recognized by the WHO and FDA. Moderna also presented crucial clinical trial data supporting the monovalent design during the June meeting – the monovalent XBB vaccine enhanced neutralizing antibodies against several XBB viral strains more effectively than the bivalent XBB and BA.4/5 combination.
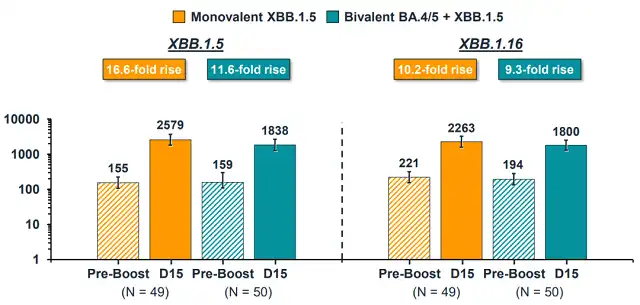
With clear data supporting the shift to monovalent XBB, the decision was made at the June expert meeting that all vaccine manufacturers would need to develop monovalent XBB vaccines for the upcoming fall and winter season. This approach is similar to that of influenza vaccines, where the FDA (usually following WHO recommendations) determines the antigen used for vaccines in the United States, and manufacturers adhere to these standards.
Of course, the pattern of COVID-19 transmission differs from influenza in some ways; it follows its own pattern of peaks and valleys in mutations. Therefore, the updated COVID-19 vaccines are expected to be available for the fall and winter season, focusing on the potential challenges of indoor gatherings and increased respiratory virus transmission during that period. Particularly concerning is the possibility of a concurrent peak in cases of traditional respiratory viruses such as influenza and RSV, which could further strain the healthcare system. Consequently, the updated COVID-19 vaccines will be aimed at the fall and winter season, rather than responding to every new variant that emerges.
As of September 11th, after undergoing the rigorous standard vaccine review process, the FDA officially approved the updated versions of the two mRNA vaccines, designed with XBB.1.5 as the template for monovalent vaccines. Some may wonder why Novavax, a manufacturer producing recombinant protein vaccines, was not mentioned since they were part of the discussions in June. The current information suggests that Novavax’s XBB.1.5 monovalent recombinant protein vaccine is still under review and will likely be available later.
This is not surprising, as large-scale production of a vaccine is not easy even for major companies like Pfizer and Moderna, and Novavax is a smaller player. Moreover, to better match the circulating viral strains, the FDA has expedited the vaccine production timeline significantly. The decision on the antigen was made in June, and the vaccines are expected to be available in September. Even with advanced design and production by all manufacturers, the large-scale production of recombinant protein vaccines takes more time, making it likely that Novavax will lag behind.
While the FDA ensures safety and efficacy, the CDC’s decision focuses on who should receive the updated vaccine. This is a crucial aspect of the discussion. Due to previous vaccinations and natural infections, the current population has a relatively high level of immunity to COVID-19, resulting in mild symptoms for most individuals after infection. This has led to differing opinions on who should continue to receive vaccines.
From a scientific perspective, anyone can benefit from receiving the updated vaccine. Even the lowest-risk, healthy young individuals can further reduce their risk of severe illness with vaccination, and the rapid increase in antibodies within a short time can greatly reduce the risk of infection. The high safety profile demonstrated by mRNA COVID-19 vaccines further solidifies the balance of benefits and risks.
However, it must be acknowledged that due to the challenges of preventing COVID-19 infection entirely, the greatest benefits of regular updates and high vaccination rates are still realized among the elderly and those with multiple underlying health conditions, who remain at significant risk of severe COVID-19. They continue to face a non-negligible risk of severe illness, and their benefits from vaccination are significantly higher than those of low-risk individuals.
Therefore, some experts at the CDC suggested that high-risk individuals should be prioritized for vaccination. However, from the perspective of simplifying the vaccination approach, allowing everyone to receive the vaccine is more convenient. Additionally, as mentioned earlier, even for the lowest-risk individuals, the benefits of vaccination outweigh the risks. In the final expert vote at the CDC, the decision was overwhelmingly in favor of recommending vaccination for all individuals aged six months and older, with a vote of 13 to 1.
Considering that the majority of people in the United States have some level of immunity due to vaccination or natural infection, the vaccination approach for the XBB.1.5 monovalent vaccine continues to be simplified: all individuals aged five and older only require one dose, while those aged five and under without a history of infection and prior vaccination require 2-3 doses.
Of course, it is difficult to predict how many people will actually get vaccinated. As of the end of May 2023, only 17% of the population had received the BA.4/5 bivalent vaccine, far below the vaccination rate for influenza vaccines. Whether the increased frequency of COVID-19 vaccine updates and vaccination approach closer to that of influenza vaccines will boost vaccination rates remains to be seen.
For individuals, the decision of whether and when to receive the vaccine can be influenced by more personalized considerations. For high-risk individuals, the high benefits of vaccination make the choice straightforward, and since COVID-19 does not have a clear peak season like the flu, it is generally advisable to get vaccinated as early as possible. The only circumstance to consider delaying vaccination is if an individual has recently recovered from COVID-19; in this case, it is advisable to wait at least two months before getting vaccinated, as the immune system is at its peak shortly after recovery, and vaccination may not have a significant impact.
As for low-risk individuals, vaccination is still advisable, but it may not be as urgent. However, if they choose not to get vaccinated, the risk is not substantial. There may be more flexibility in choosing when to get vaccinated, such as closer to the peak of winter travel and gatherings, which can maximize the short-term effectiveness of the vaccine in preventing infection. Of course, timing vaccination this way can be somewhat unpredictable since COVID-19 continues to circulate, and there’s no foolproof way to predict when someone may be exposed to the virus.
One erroneous reason for refusing the updated vaccine is the belief that XBB.1.5 is no longer the prevalent strain, and therefore, getting the XBB.1.5 vaccine is pointless. While the FDA and vaccine manufacturers aim to align the updated vaccines as closely as possible with the prevalent strain, the evolution of the COVID-19 virus makes it challenging to achieve a perfect match. However, the inability to achieve an exact match does not render the vaccine ineffective. XBB.1.5 is highly similar to the currently prevalent EG.5 and even the more recent and significantly different BA.2.86. Evidence suggests that the XBB.1.5 monovalent vaccine provides protection even against these variants, with no significant immune escape observed.
The updated XBB.1.5 monovalent mRNA vaccines have ample data supporting their efficacy and safety, providing protection against various viral strains currently in circulation. This is a fact and, in my opinion, the only consideration for anyone hesitating to get vaccinated.
CDC Issues Updated Recommendation for XBB.1.5 COVID-19 Vaccine
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



