How to understand the data behind the bivalent COVID-19 vaccine approved by U.S.?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
How to understand the data behind the bivalent COVID-19 vaccine approved by U.S.?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
How to understand the data behind the BA.4/5 bivalent COVID-19 vaccine approved by U.S. ?
On August 31, the FDA approved the updated COVID-19 vaccines of Pfizer/BioNTech and Moderna, both of which are bivalent designs of the original vaccine + BA.4/5 vaccines.
The ACIP of the CDC on September 1 also recommended this design. reinforced needle. These recommendations are based on previous clinical trial experience of the BA.1 vaccine plus data from the BA.4/5 vaccine in an animal model – mice.
Based on these data, the FDA believes that there is sufficient evidence that the BA.4/5 bivalent vaccine is superior to the original version and the BA.1 version that was previously in clinical trials.
Of course, in the absence of human data for the BA.4/5 bivalent vaccine, it is controversial whether the mouse data can be used to infer the performance of this vaccine in humans.
At the CDC’s ACIP meeting, two mRNA vaccine manufacturers announced relevant animal experimental data, giving us a chance to see how good the so-called better is.
1. Moderna’s BA.4/5 vaccine data
Moderna’s mouse experiment is based on two injections of the original vaccine mRNA-1273, and booster injections are given every 31 weeks.
The booster injections are the original vaccine mRNA-1273 , BA.1 bivalent mRNA-1273.214 and BA.4 /5 bivalent mRNA-1273.222 , while a group of mice was given PBS as a placebo control [1].
The result is as follows:
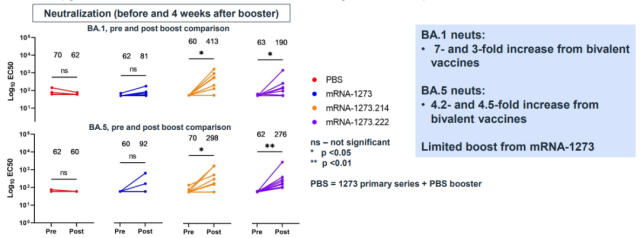
The study only compared BA.1 and BA.5 neutralizing antibody titers four weeks after vaccination.
If looking at the titer of BA.1, the BA.1 bivalent vaccine performed better than the BA.5 bivalent vaccine (the titers after vaccination were 413 and 190, respectively).
The BA.5 titers were comparable (298 vs. 276).
From this result of Moderna, it is difficult to understand how to draw the conclusion that BA.4/5 bivalent vaccine is better than BA.1 bivalent.
What is even more surprising is that the original version of the vaccine did not have any enhancement effect – the third dose of the original version of the vaccine did not increase the neutralizing antibodies of the two Omicron substrains.
This result is completely different from previous clinical trials and real-world observations.
Even in the clinical trial of the BA.1 bivalent vaccine, the original version of the vaccine as a control still enhanced the neutralizing antibody of BA.1 [2]:
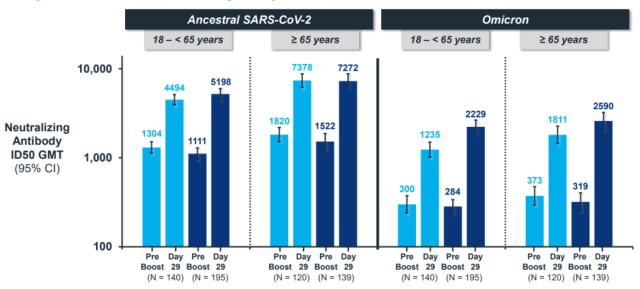
Moderna’s BA.4/5 bivalent vaccine mouse experiment is the third comparison.
If the original vaccine data in the experiment is correct, that is, the third injection did not increase the neutralization effect of Omicron, then the Moderna booster injections all over the world have been lonely?
A more plausible explanation would be that for some unknown reason, the original vaccine failed in this mouse trial.
For anyone working in a lab, it’s not much of a surprise that experiments fail. The key is how to interpret the data when these situations arise.
Although Moderna’s experiment is to study two Omicron bivalent vaccines, the original vaccine is an important control and an important link to connect the results of the mouse experiment to the real world – the vast majority of people in the world are vaccinated with the original vaccine. version vaccine.
So important controls have not been made, how should the results of the entire experiment be interpreted, or even can it be interpreted? is to be suspicious.
For anyone who has undergone rigorous experimental science training, a basic principle is: any experiment as long as the results of the control group are incorrect, no matter what the results of the experimental group are, it is unacceptable.
In addition to neutralizing antibodies, Moderna also conducted a challenge experiment to verify the protective effect of the vaccine [1]:
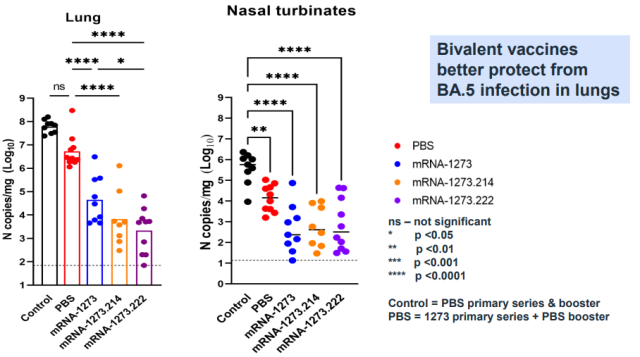
Likewise, there is no difference between BA.1 bivalent and BA.4/5 bivalent in this experiment, again making the hypothesis that BA.4/5 bivalent vaccine better is questionable.
For the protection of the lungs, the two Omicron bivalents are better than the original version of the vaccine, but the original version of the vaccine did not even increase the antibody in this experiment.
Can it be used as a control to prove that the updated version of the vaccine is better?
Even more inexplicably, the PBS placebo group (vaccinated with two original vaccines and not vaccinated with the third dose) and the control (mice not vaccinated with any vaccine) had differences in the amount of virus in the nasal cavity and the amount of virus in the lungs. But no difference.
If the effectiveness of the vaccine declines, the first decline is the prevention of mild symptoms, which corresponds to the deterioration of the upper respiratory tract protection.
How can it become the first loss of protection in the lungs?
If this result is put into the real world, it means that people who have been vaccinated with two vaccines and have not received a booster shot will not have a lower risk of severe illness after infection than those who have not received a single shot of the vaccine.
There are too many unjustifiable and unrealistic situations in this experimental result, and I am not convinced that such results are suitable for confirmation of the effectiveness of the updated vaccine.
2. Pfizer/BioNTech bivalent vaccine data
In addition to Moderna, Pfizer/BioNTech also showed mouse data, in which the experiment comparing the BA.4/5 bivalent vaccine is probably the only one published in the world so far that can be used to prove that the BA.4/5 bivalent is better The data[3]:
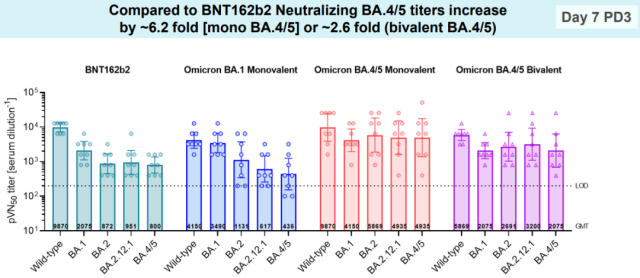
In the experiment, mice were vaccinated 104 days after two doses of the original BNT162b2 vaccine: the original version, BA.1 monovalent, BA.4/5 monovalent and BA.4/5 bivalent.
If you look at the neutralizing antibody of BA.4/5 (one week after the third injection), the bivalent of BA.4/5 is 2.6 times higher than the original version, and the unit price of BA.4/5 is 6.2 times higher.
This seems to indicate that BA.4/5 bivalent is superior to the original vaccine, but there is no BA.1 bivalent in this experiment, so it cannot explain the abandonment of the previous BA.1 bivalent and the choice of BA.4/5 without human data.
How much advantage does the second price bring?
More critically, the experiment also had many incomprehensible parts. According to this experiment, BA.4/5 monovalent is the best vaccine:
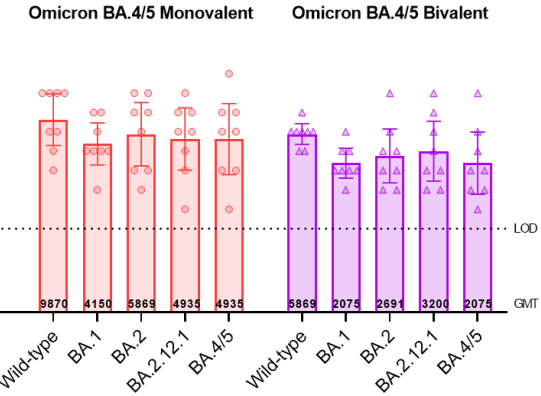
Compared with bivalent, BA.4/5 monovalent has more neutralizing antibodies to the original virus and each substrain of Omicron (basically about twice).
If the neutralizing antibody of bivalent to BA.4/5 is more than 2 times higher than that of the original vaccine, it is enough to convince us that we should switch to bivalent, and the unit price of BA.4/5 is more than 2 times higher than that of bivalent, then why Why not replace this monovalent vaccine?
It’s not that the unit price BA.4/5 is really better than the second price, but I just want to show that the results of this experiment are difficult to be self-consistent .
Especially for the neutralizing antibody titer of the original virus strain, the monovalent of the original vaccine and BA.4/5 is 9870, while the bivalent of BA.4/5 is only 5869, which is lower than both.
The bivalent is obviously two and a half. Since the unit price of BA.4/5 is not worse than the original vaccine, how could the bivalent become worse in the end?
At the same time, the performance of BA.1 monovalent in this experiment was surprisingly poor, the neutralizing antibody titer to BA.4/5 was only half of that of the original strain, and the increase of BA.1 antibody was less than 2 times.
Looking at this result, it seems that I should be glad that BA.1 was not replaced – of course, BA.1 is actually a two-price rather than a unit price.
In another mouse experiment, the performance of BA.1 monovalent is not the same [3]:
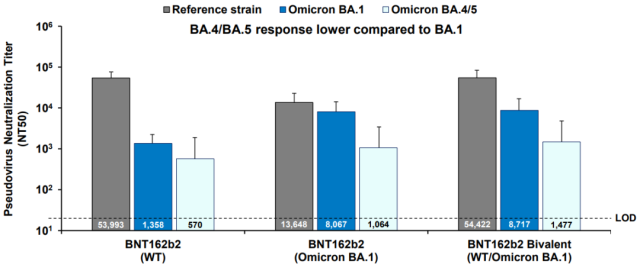
How to understand the data behind the BA.4/5 bivalent COVID-19 vaccine approved by U.S. ?
It is also the third injection after two injections of the original vaccine, but the interval is shortened to one month.
In this experiment, the BA.1 monovalent neutralizing antibody to BA.1 is 6 times higher than that of the original vaccine, and the neutralizing antibody to BA.4/5 is also higher. nearly 2 times.
The same vaccine is so different in the two experiments, does it reflect the poor stability of these experiments themselves, and it is difficult to test the pros and cons of the updated vaccines with only slight differences in composition?
3. Data, conclusions and expectations need to be reconciled
As the differences between the mainstream mutant strains and the original virus strains become larger and the immune escape becomes more and more significant, it is no problem to update the vaccines based on the original virus strains to make the vaccines antigenically closer to the currently popular mutant strains.
But the actual effect of the updated vaccine needs to be based on sufficient data to draw conclusions , and the expected effect communicated to the public must be consistent with the data .
Updating the BA.4/5 bivalent vaccine, the question is not just whether the BA.4/5 vaccine is useful, whether it can be used as a booster to increase antibodies.
From the clinical trial results of various mutant vaccines in the past – including the original vaccine, beta strain vaccine, and BA.1 vaccine, we can say with great confidence that on the basis of previous vaccination with the original vaccine, BA.4/ 5 The bivalent vaccine will increase the antibodies that have declined in a short period of time, and will have the effect of enhancing the needle.
In particular, half of the bivalent vaccines are still the original vaccines. If there is no enhancement effect, it would be hell.
Updating to BA.4/5 means replacing the original vaccine as a booster, the evidence needed, and the question we are asking is: Is the BA.4/5 bivalent vaccine significantly better than the original vaccine .
In addition, there was a BA.1 bivalent vaccine before – which has been approved by the United Kingdom and Switzerland, and it has been extended to determine whether it is better than the BA.1 vaccine.
But whether it is Moderna or Pfizer/BioNTech, does there really have data to clearly answer the above questions?
Neither had human data, so it was assumed that mouse experiments would adequately predict human outcomes.
But is it really so? The third injection of the original vaccine in Moderna’s mouse experiments did not enhance the effect, and the BA.1 vaccine in Pfizer/BioNTech was extremely ineffective.
Are these consistent with past human data?
Not to mention that there is no difference between BA.4/5 bivalent and BA.1 bivalent in Moderna’s mouse experiment, and BA.4/5 monovalent is better than bivalent in Pfizer/BioNTech test, how can we deduce BA. 4/5 two price is better?
There are also details, such as Moderna’s experiments with groups of 8-10 mice.
Regardless of whether this amount of data is enough to extend to tens of millions of people, some experimental groups have only 3 points in the neutralizing antibody titer test, that is, three mice.
Are the remaining rats refusing to donate blood or have they escaped?
There seems to be a general optimism about the updated vaccine now – both China and the United States, there are assumptions that updating to BA.4/5 will be more effective.
Both the FDA and the CDC have mentioned that the updated vaccine may restore the anti-infection effect, but are such expectations reasonable?
Regardless of the various defects in animal experiments, the only Pfizer/BioNTech experiment that showed that BA.4/5 bivalent is better than BA.1 bivalent, BA.4/5 bivalent vaccine is higher than the original vaccine by BA.4/5.
And the antibody is only 2.6 times. In clinical trials, the BA.1 bivalent mRNA vaccine improved BA.1 neutralizing antibodies only about 1.7 times higher than the original vaccine.
Can these 2-3 times differences really bring about a qualitative leap? The initial half-life of neutralizing antibodies in the human body is about 1 month, which is increased by 2-3 times, and returns to the original point after a month.
Can this limited, short-term increase in antibodies really be expected to provide a good blockade of infection?
A more realistic expectation should still be protection against severe illness and death, which is the same as the current original vaccine.
Expectations for the BA.4/5 vaccine should return to rationality based on data rather than blind optimism.
References :
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/06-COVID-Miller-508.pdf
https://www.fda.gov/media/159492/download
https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/07-COVID-Swanson-508.pdf
How to understand the data behind the bivalent COVID-19 vaccine approved by U.S.?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



