UK approved the world’s first vaccine for COVID-19 Omicron
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
UK approved the world’s first vaccine for COVID-19 Omicron
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
UK approved the world’s first vaccine for COVID-19 Omicron.
On August 15, the United Kingdom became the first country in the world to approve the Omicron vaccine [1].
Moderna’s bivalent original version + Omicron BA.1 vaccine received the green light from the British government and became the world’s first approved next-generation COVID-19 vaccine.
1. Not waiting for BA.5 version
The most notable point of the UK approval is that it was approved for the Omicron vaccine against BA.1 .
In June, Moderna and Pfizer/BioNTech respectively announced the clinical trial results of the BA.1 version of the vaccine.
However, the current mainstream new coronavirus strain is no longer the BA.1 substrain that swept the world at the end of 2021 and early 2022, but BA.5.
This brings about a trouble: whether to choose the BA.1 version with clinical trial data, or take a gamble and switch to BA.5 directly – which means that it is too late to get the clinical trial data.
The decision made by the FDA at the end of June was a gamble, requiring vaccine companies to update the COVID-19 booster shot this autumn and winter to the original vaccine + BA.5 bivalent vaccine.
But before the FDA decision, WHO has approved the addition of BA.1 version to the booster needle. The idea of WHO has also been recognized by some European countries.
The FDA later acknowledged that different countries may take different routes, including the use of the BA.1 vaccine. And this British approval can be said to make this different route a reality.
Of course, on the other hand, the approval of BA.1 this time is not immediately enabled, and the actual use will still be in late September .
For the population, only people over 50 years old or immunosuppressed are listed.
If the BA.5 version of the vaccine is available by then, it is unknown whether the UK will still use the BA.1 bivalent approved today.
Therefore, the UK’s decision today can be said to be neither planning to wait, but still waiting for the BA.5 vaccine.
2. Reasons for changing BA.1
From the perspective of antigen recognition of the immune system, the sub-strains of Omicron have the most antigenic differences from the original strains used in today’s vaccines .
This is the root cause of Omicron’s strong immune escape from existing vaccines.
However, taking advantage of this antigenic difference and selecting Omicron as a model to change the composition of the vaccine’s virus strains, it is also more hopeful to improve the breadth of the immune response , not only to improve the protection of existing mutant strains, but also to better cope with new vaccines in the future.
The immune response studies of some breakthrough infections also suggest that it is feasible to increase the breadth of the immune response by relying on the Omicron antigen [2]:
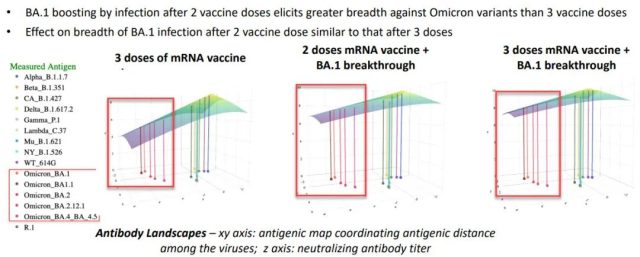
After two or three doses of the original version of mRNA, the BA.1 breakthrough infection improved the breadth of the immune response better than the third dose of the original version of the vaccine – each substrain of Omicron in the red box was neutralized Antibody titers were higher and closer to the other mutants.
Judging from the clinical trial results of the Omicron BA.1 version of the vaccine announced by Moderna and Pfizer/BioNTech, it also confirmed that the BA.1 version of the vaccine improved the breadth of the immune response as expected.
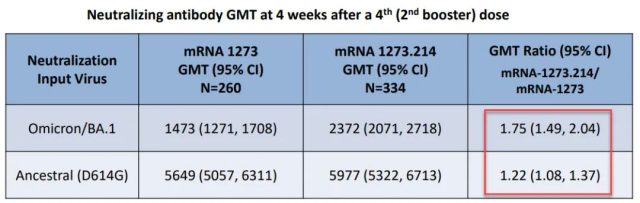
Like the bivalent version of the original Moderna vaccine + BA.1 vaccine approved in the UK this time, compared with the original vaccine, in the clinical trial of the second booster needle, the neutralizing antibody values are compared [2]:
Compared with the original vaccine, the updated bivalent vaccine has more than 1.7 times higher antibodies to BA.1, and similar antibodies to the original virus strain.
This achieves the purpose of updating the vaccine: to increase the breadth of immune recognition while maintaining the strength of the immune response .
The approval of the BA.1 version of the vaccine in the UK this time is also a recognition of the results of these clinical trials of Moderna.
It is believed that the next-generation vaccine of BA.1 has a better immune response breadth than the original vaccine.
In addition, the BA.1 version vaccine of Moderna and Pfizer/BioNTech is currently the updated version of the vaccine with the most actual data.
Compared with the BA.5 version, this version has the least unknown and the most known.
3. Should I wait for BA.5?
But today, when BA.1 is almost extinct, the approval of BA.1 is bound to face the question of why it does not wait for the BA.5 version of the vaccine.
The inescapable reality is that the immune escape features of BA.5 are different and more severe than those of BA.1 .
Even from the clinical trial of Moderna, although the BA.1 bivalent vaccine has greatly improved the neutralizing antibody against BA.5, the value is much lower than the antibody against BA.1 [3]:
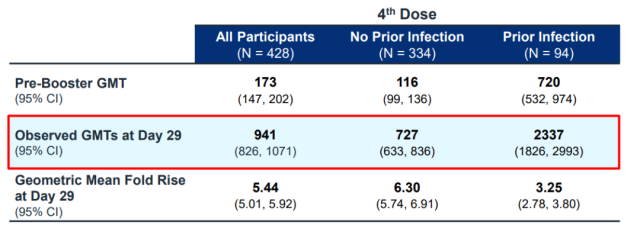
For subjects without previous infection, after receiving the BA.1 bivalent vaccine (the second booster shot), the neutralizing antibody titer to BA.5 is 727, which can exceed 2300 to BA.1 in the previous table .
This BA.5 antibody is only one-fourth to one-third of the BA.1 antibody, which is a common phenomenon.
If an updated version of the vaccine is designed according to BA.5, will it have a better effect on the currently circulating mutant strain-BA.5?
This is something that European and American drug regulatory structures need to consider.
The ideal situation is naturally to complete the clinical trial of the BA.5 vaccine and directly compare it with the BA.1 vaccine. But the reality is that autumn is just around the corner.
In order to catch up with the time line of the autumn booster, the approval and launch of the updated booster must be earlier than the clinical trial results of the BA.5 version of the vaccine .
That is to say, the so-called waiting for the BA.5 version of the vaccine, the actual waiting is not to confirm that BA.5 is better than BA.1, but to assume that BA.5 is better than BA.1, and waiting is only for the production of BA.5 version.
4. Wait or wait, maybe it’s almost the same
In fact, the difference between the original mRNA vaccine, BA.1 and BA.5 is not that big.
The final main role will be the protection of severe cases, and the effectiveness of preventing infection may be relatively limited .
Judging from the BA.1 version with the most current data, the comparison of neutralizing antibodies in Moderna’s clinical trials of nearly 600 people [3]:
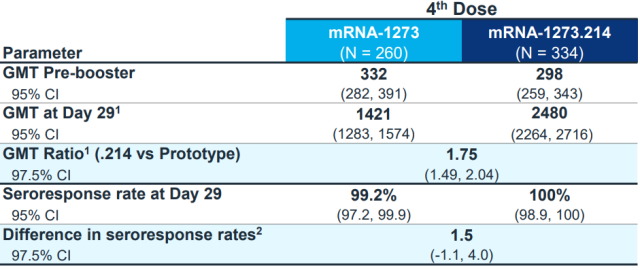
The BA.1 bivalent vaccine approved in the UK has only 1.75 times higher antibodies against BA.1 than the original vaccine.
It’s higher, but does this increase lead to a sea change in effectiveness?
No optimistic scientist would not think so. The BA.5 version of the vaccine is likely to be similar.
Perhaps one of the biggest advantages of approving the BA.1 version of the vaccine as in the UK is the flexibility .
In parallel with the clinical trials of the BA.1 vaccine, Moderna and Pfizer/BioNTech are both “risking” the production of the BA.1 vaccine.
At the FDA vaccine update expert meeting in June, both companies said that the BA.1 version of the vaccine would accumulate a lot in August, enough to carry out a new round of booster vaccinations.
In contrast, the BA.5 vaccine will not be available until late September because of its late start of production.
With the ongoing uncertainty of the epidemic – such as whether there will be a new mutant to replace BA.5, and whether there will be a more severe infection peak in the autumn, giving yourself more options, this may be the UK’s lead A small calculation for the world’s approval of the Omicron vaccine.
But on the other hand, since the United States has decided to update to BA.5, considering the largest market in the United States, the decision is decisive for European and American vaccine manufacturers.
It is hard to imagine that there are pharmaceutical companies, including two mRNA vaccine manufacturers, that do not go all out to produce the BA.5 version of the vaccine.
For most countries, whether they want to wait for the BA.5 vaccine or not, they can only participate in the waiting.
References :
https://www.theguardian.com/world/2022/aug/15/dual-variant-covid-vaccine-approved-uk-booster-programme-moderna
https://www.fda.gov/media/159495/download
https://www.fda.gov/media/159492/download
UK approved the world’s first vaccine for COVID-19 Omicron
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



