NIH Launches Clinical Trial for “Ultimate Influenza Vaccine” FluMos-v2
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
NIH Launches Clinical Trial for “Ultimate Influenza Vaccine” FluMos-v2
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
NIH Launches Clinical Trial for “Ultimate Influenza Vaccine” FluMos-v2.
The first-phase trial of the universal influenza candidate vaccine, FluMos-v2, has been initiated at the National Institutes of Health Clinical Center in Bethesda, Maryland.
Sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), this trial aims to assess the safety and potential immunogenicity of the vaccine.
FluMos-v2 is an upgraded version of FluMos-v1, designed to provide broader immunity against six different strains of influenza viruses.
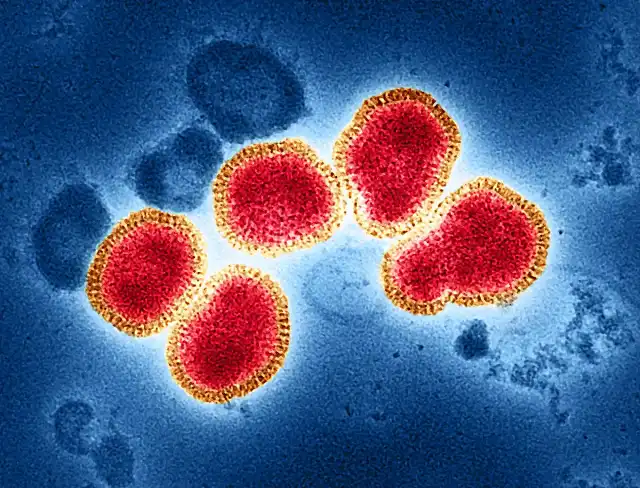
Colored transmission electron micrograph (red and gold) of influenza A virus particles isolated from a patient sample and then propagated in cell culture.
Influenza A can infect humans as well as animals such as birds and pigs. Source: National Institutes of Health
Colored transmission electron microscopy image of isolated particles of influenza A virus (in red and gold) obtained from patient samples and then propagated in cell culture. Influenza A viruses can infect humans as well as birds and other animals. Source: National Institutes of Health, USA.
The NIH Clinical Center, located in Bethesda, Maryland, has initiated a Phase 1 clinical trial for a novel universal influenza candidate vaccine. This trial, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), will evaluate the safety of the investigational vaccine and its ability to induce an immune response.
Existing Influenza Vaccines
Currently, existing seasonal influenza vaccines are effective in preventing specific strains of the flu. These vaccines are reevaluated and modified each year to best match the most predominant flu virus strains expected in the upcoming flu season. Most seasonal influenza vaccines are designed to train the immune system to defend against three to four different common flu viruses, but “universal” influenza vaccines may one day provide protection against a broader range of flu viruses.
“The ideal universal influenza vaccine would require fewer vaccinations than once a year and offer protection against multiple strains of the flu. With each new candidate and clinical trial for universal influenza vaccines, we are getting closer to that goal,” said Dr. Hugh Auchincloss, Acting Director of NIAID.
About the Candidate Vaccine FluMos-v2
The candidate vaccine under investigation, FluMos-v2, is designed by researchers at the Vaccine Research Center (VRC) of NIAID. FluMos-v1 began its first human trials in 2021 and is still in progress. FluMos-v2 aims to induce antibodies against multiple different strains of influenza viruses by displaying portions of the influenza hemagglutinin (HA) protein in a repetitive pattern on self-assembling nanoparticle scaffolds. Exposure to these harmless viral protein fragments allows the immune system to recognize and combat the actual viruses. In animal experiments, the investigational vaccine generated robust antibody responses.
While FluMos-v1 displayed the HA of four influenza virus strains, FluMos-v2 displays the HA of six influenza virus strains, including four influenza A strains and two influenza B strains. Researchers anticipate that this will further enhance immunity among vaccine recipients, providing protection against a wider array of influenza viruses.
Trial Details and Next Steps
The new clinical trial is expected to enroll 24 healthy volunteers between the ages of 18 and 50 who will receive two intramuscular injections of the FluMos-v2 candidate vaccine, with a 16-week interval between injections. Participants will initially be enrolled in a low-dose group (60 micrograms per injection). If at least three participants show no safety issues after receiving this dose of the vaccine, recruitment will commence for the high-dose group (180 micrograms per injection). The research team plans to enroll 12 participants in each dose group.
Within 40 weeks following the first vaccine injection, participants will undergo regular phone follow-ups and examinations to monitor their response to the investigational vaccine. Blood samples will also be collected during visits to measure the immune response to the candidate vaccine.
NIH Launches Clinical Trial for “Ultimate Influenza Vaccine” FluMos-v2
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



