Semaglutide: A potent treatment for chronic kidney disease
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
Semaglutide: A potent treatment for chronic kidney disease, sees remarkable clinical outcomes as Novo Nordisk halts Phase 3 trials prematurely.
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Semaglutide: A potent treatment for chronic kidney disease, sees remarkable clinical outcomes as Novo Nordisk halts Phase 3 trials prematurely.
In June 2021, the FDA approved Novo Nordisk’s GLP-1 receptor agonist, Semaglutide, as a weight loss medication under the brand name Wegovy.
Due to its exceptional weight loss effects, favorable safety profile, and endorsement by celebrities like Elon Musk, Semaglutide has gained immense popularity worldwide.
According to Novo Nordisk’s 2022 annual financial report, Semaglutide achieved a staggering $12 billion in sales during 2022.
In addition to its use for blood sugar management and weight loss, recent research suggests that Semaglutide offers unexpected benefits, including restoring the functionality of natural killer cells (NK cells) to reduce cancer risk, lowering the risk of cardiovascular diseases, and assisting in smoking and alcohol cessation, among others.
On October 10, 2023, Novo Nordisk announced the premature termination of Phase 3 clinical trials for Semaglutide’s treatment of kidney damage in patients with type 2 diabetes and chronic kidney disease.
This decision was based on the recommendation of an independent data monitoring committee, which concluded that the mid-term analysis results met certain predefined criteria, allowing for the trial’s early termination.

In light of this decision, Novo Nordisk will initiate the procedures to conclude the clinical trial. To maintain the trial’s integrity, Novo Nordisk will keep the results blinded until the trial is completed, with an expected release date in the first half of 2024.
The clinical trial, named FLOW, is a randomized, double-blind, parallel-group, placebo-controlled, superiority trial that compares the effects of Semaglutide 1.0mg injections as an adjunct treatment to standard care for preventing kidney outcomes in patients with type 2 diabetes and chronic kidney disease (CKD). A total of 3,534 patients participated in this trial, which was conducted in 418 research sites across 28 countries and initiated in 2019.
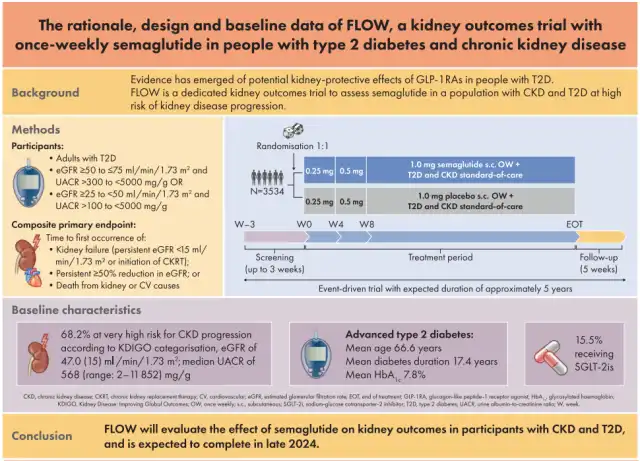
Chronic kidney disease (CKD) is a common complication of type 2 diabetes (T2D). Glucagon-like peptide-1 receptor agonists (GLP-1RAs) can improve blood sugar control and reduce weight in T2D patients, and they also lower the risk of cardiovascular events in high cardiovascular risk populations. GLP-1RAs may also have a kidney-protective effect. This clinical trial aimed to investigate the impact of Semaglutide (administered once weekly at 1.0mg per dose) on kidney outcomes in CKD and T2D patients.
The FLOW trial includes several key composite endpoints, such as eGFR1 continuously decreasing by ≥ 50% from baseline, time with eGFR1 <15 mL/min/1.73m², time to the initiation of chronic kidney replacement therapy (dialysis or kidney transplant), rates of kidney disease-related or cardiovascular disease-related deaths in T2D and CKD patients, to demonstrate the delay in the progression of chronic kidney disease by Semaglutide and the reduction in the risk of kidney and cardiovascular disease-related deaths. Key secondary endpoints encompass the annual change rate in eGFR1, major adverse cardiovascular events (non-fatal myocardial infarction, non-fatal stroke, cardiovascular death), and the risk of all-cause mortality. The trial protocol stipulated a mid-term analysis when a predefined number of primary endpoints were reached.
References:
https://doi.org/10.1093/ndt/gfad009
https://www.globenewswire.com/news-release/2023/10/10/2757941/0/en/Novo-Nordisk-will-stop-the-once-weekly-injectable-semaglutide-kidney-outcomes-trial-FLOW-based-on-interim-analysis.html(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



