Semaglutide May Pose Serious Gastrointestinal Risks
- Gut Bacteria Enzymes Offer Hope for ABO Universal Blood Transfusions
- Well-Known Japanese Medicine Exposed for 30 Years of Data Falsification
- Oregon Reverses Course: From Decriminalization to Recriminalization of Drug Possession
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
JAMA: “Weight Loss Wonder Drug” Semaglutide May Pose Serious Gastrointestinal Risks
- Was COVID virus leaked from the Chinese WIV lab?
- HIV Cure Research: New Study Links Viral DNA Levels to Spontaneous Control
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
JAMA: “Weight Loss Wonder Drug” Semaglutide May Pose Serious Gastrointestinal Risks.
Glucagon-like peptide-1 (GLP-1) receptor agonists, approved for the treatment of type 2 diabetes due to their ability to curb appetite and reduce food intake, including drugs like semaglutide and liraglutide, have gained prominence in the weight loss community for their remarkable effectiveness.
Previous research has indicated an elevated risk of gastrointestinal adverse events in diabetes patients using GLP-1 drugs, including gallbladder disease, pancreatitis, intestinal obstruction, and gastroparesis. Currently, it remains unclear whether the use of these drugs for weight loss also increases the risk of gastrointestinal adverse events.
On October 5, 2023, researchers from the University of British Columbia’s Faculty of Medicine published a study titled “Risk of Gastrointestinal Adverse Events Associated With Glucagon-Like Peptide-1 Receptor Agonists for Weight Loss” in the Journal of the American Medical Association (JAMA).
The study reveals that using GLP-1 agonists for weight loss raises the risk of pancreatitis, gastroparesis, and intestinal obstruction. Compared to non-GLP-1 weight loss medications, the risk of pancreatitis increases 9.1 times, intestinal obstruction risk increases 4.22 times, and gastroparesis risk increases 3.67 times.

In this study, researchers randomly selected samples from 16 million patients in the PharMetrics Plus database (IQVIA). They analyzed a total of 5,411 non-diabetic participants, including 4,144 using liraglutide, 613 using semaglutide, and 654 using phentermine-topiramate (a non-GLP-1 weight loss drug) in clinical settings, focusing on gastrointestinal adverse events associated with GLP-1 agonists for weight loss.
During the follow-up period of the study, participants using semaglutide, liraglutide, and phentermine-topiramate had pancreatitis incidence rates per 1,000 person-years of 4.6, 7.9, and 1, respectively; intestinal obstruction incidence rates were 0, 8.1, and 1.7, respectively; and gastroparesis incidence rates were 9.1, 7.3, and 3.1, respectively. However, gallbladder disease incidence rates were 11.7, 18.6, and 12.6, respectively.
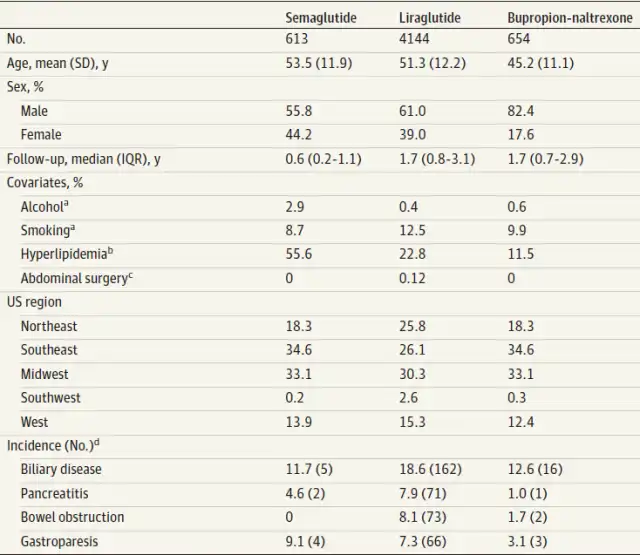
The study findings indicate an increased risk of pancreatitis, gastroparesis, and intestinal obstruction with the use of GLP-1 agonists for weight loss but not an increased risk of gallbladder disease.
Specifically, participants using GLP-1 agonists had a 9.1-fold increased risk of pancreatitis, a 4.22-fold increased risk of intestinal obstruction, and a 3.67-fold increased risk of gastroparesis compared to those using phentermine-topiramate.
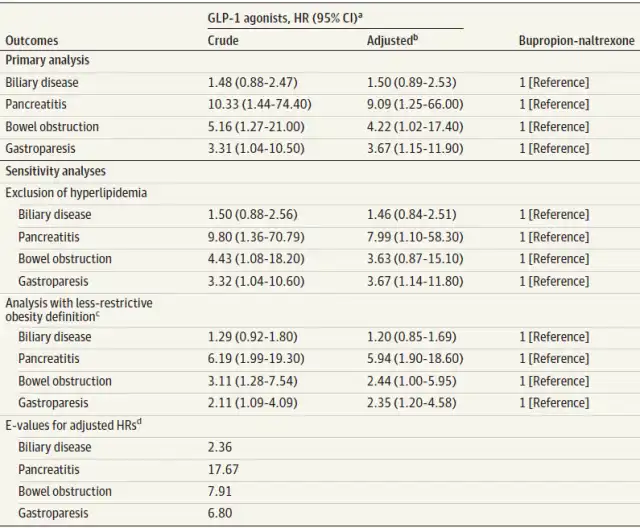
Researchers suggest that while these adverse events are rare, given the widespread use of these drugs, patients considering the use of these medications for weight loss should take these risks into account. With the growing popularity of these drugs, hundreds of thousands of people worldwide may be at risk.
It’s worth noting that on September 22, 2023, the U.S. FDA updated the label for the diabetes drug Ozempic, acknowledging cases of intestinal obstruction in some users.
The FDA database disclosed 8,571 reports of gastrointestinal diseases related to semaglutide drugs, including Ozempic and Wegovy. Among these reports, 33 specifically mentioned intestinal obstruction reactions, resulting in 2 deaths.
Additionally, some users of Ozempic and Wegovy also reported a condition known as gastroparesis.
In summary, this study indicates that the use of GLP-1 agonists for weight loss increases the risk of pancreatitis, gastroparesis, and intestinal obstruction compared to the use of phentermine-topiramate. While adverse events are rare, patients using these drugs for weight loss must consider these risks.
JAMA: “Weight Loss Wonder Drug” Semaglutide May Pose Serious Gastrointestinal Risks
Paper Link:
DOI: 10.1001/jama.2023.19574
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



