Immune Checkpoint Receptor Signaling Pathways in T Cells
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
Immune Checkpoint Receptor Signaling Pathways in T Cells
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Immune Checkpoint Receptor Signaling Pathways in T Cells
The rapid development of research on receptors that negatively regulate lymphocyte function is driven by the success of tumor immunotherapy.
While much research focuses on characterizing these immune checkpoint receptors from a functional perspective, there is relatively less emphasis on studying their signaling mechanisms.
Current research has demonstrated that the extracellular portions of some receptors act as bait receptors for activating ligands, while in most cases, the phosphorylation of tyrosine residues in their cytoplasmic tails drives critical inhibitory signals.
These negative signals are mediated by key signaling transducers, such as tyrosine kinases, inositol phosphatases, and diacylglycerol kinases, allowing them to counteract T-cell receptor (TCR)-mediated activation.
The features of these signaling pathways are crucial for developing new immunotherapies and overcoming the limitations of current immune checkpoint inhibitors.
T-Cell Signaling Pathways
T-cell signaling mechanisms primarily facilitate the rapid expansion, differentiation, and effector response of T lymphocytes when recognizing non-self antigens presented by antigen-presenting cells (APCs). This extraordinary discriminatory ability arises from the integration of activating and inhibitory signals within the cell.

The first activation signal is delivered by the T-cell receptor (TCR), which recognizes antigens bound to the major histocompatibility complex (MHC) on APCs. Activated TCR triggers the assembly of a signalosome, with key components including tyrosine kinases like Lck and Zap70, scaffold proteins such as Lat, SLP76, and Themis, and phospholipase Cγ1 (PLC γ1), as well as tyrosine phosphatases and E3 ubiquitin ligases like SHP1 and Cbl, among others.
The second activation signal for T cells is provided by co-stimulatory receptors like CD28. CD28 enhances TCR-driven tyrosine phosphorylation and interacts with phosphatidylinositol 3-kinase (PI3K) and Grb2, initiating the Akt-mTOR and Ras-MAPK pathways.
The most characteristic inhibitory receptors for lymphocytes are PD-1 and CTLA-4, which represent examples of T-cell inhibitory receptors. Both continuously suppress cytokine secretion and proliferation induced by TCR, as well as glucose uptake and metabolism. The cytoplasmic tails of these inhibitory receptors contain tyrosine-based inhibitory motifs (ITIMs) and switch motifs (ITSMs) with common sequence features. These motifs are phosphorylated by Src family kinases (SFKs) upon receptor activation and play crucial roles in regulating the immune system. ITIMs negatively modulate cell activation by recruiting phosphatases such as protein tyrosine phosphatases 1 and 2 (SHP-1 and SHP-2), as well as inositol 5′-phosphatases 1 and 2 (SHIP-1 and SHIP-2). ITSMs can transmit positive or negative signals by recruiting adapters like signaling lymphocytic activation molecule (SLAM)-associated protein (SAP).
PD-1 Signaling Pathway
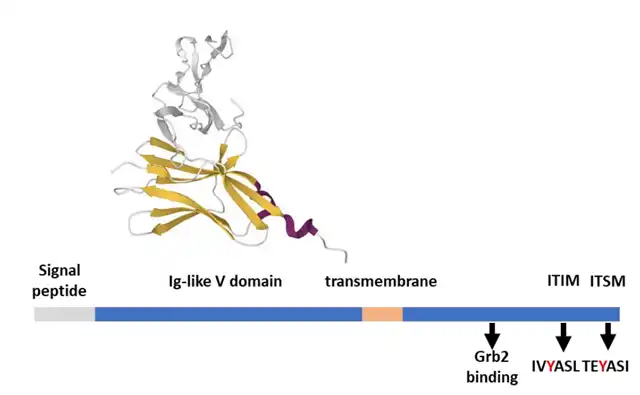
Programmed cell death protein 1 (PD-1, CD279) is a transmembrane glycoprotein that belongs to the CD28 receptor superfamily. Unlike CD28, PD-1 exists as a monomer on the cell surface. Structurally, PD-1 consists of extracellular immunoglobulin (Ig)-like variable domains, a transmembrane domain, and a cytoplasmic tail responsible for signal transduction and binding to scaffold molecules. The cytoplasmic tail of PD-1 contains two tyrosine-based motifs, an ITIM (VDY223GEL), and an ITSM (TEY248ATI). Both motifs are phosphorylated by the tyrosine kinase Lck when PD-1 ligands bind to PD-1.

PD-1 is expressed on activated T cells, B cells, natural killer (NK) cells, monocytes, dendritic cells, and cancer cells such as melanoma. PD-1 is activated through its interaction with PD-L1 (B7-H1, CD274) and exhibits higher affinity for PD-L2 (B7-DC, CD273). Both ligands are induced by interferon/cytokines but have distinct expression patterns: PD-L1 is broadly expressed in hematopoietic and non-hematopoietic cells, while PD-L2 is primarily expressed on APCs.
In immune cells, PD-1 signaling relies on tyrosine phosphatase SHP-2. Disruption of the PD-1/SHP-2 signaling axis is partly responsible for the clinical response of PD-1 antibodies in the tumor microenvironment. After ligand binding, SHP-2 is recruited to the phosphorylated ITSM of PD-1. Phosphorylation of ITSM induces a conformational change in SHP-2 towards an active state. Microscopy studies of reconstructed immune synapses show that in the presence of PD-L1, PD-1 and CD28 associate in the central TCR-enriched area. PD-1 recruits SHP-2, promoting the dephosphorylation of CD3ζ and CD28 and negatively affecting TCR signaling strength. PD-1-mediated CD28 dephosphorylation significantly impacts PI3K recruitment at the TCR signalosome, reducing PI3K/AKT pathway activity and its downstream transcriptional targets, such as Bcl-xL. Furthermore, SHP-2 is thought to not only block CD28 co-stimulatory signals but also inhibit TCR-mediated ZAP70 phosphorylation and its association with CD3ζ, which leads to PKCθ and ERK activation, as well as downstream IL-2 production and amplification.
CTLA-4 Signaling Pathway
Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) interacts with the co-stimulatory ligands B7-1 (CD80) and B7-2 (CD86) with higher affinity than CD28 itself. These interactions yield CTLA-4’s inhibitory function by competing with CD28 for ligands, thus reducing the second signal required for full T-cell activation. Additionally, CTLA-4 constitutively internalizes ligands on APCs, decreasing their T-cell activating ability.
Structurally, CTLA-4 shares extensive homology with CD28. Their extracellular portions have immunoglobulin-like V domains, allowing the formation of disulfide-bonded homodimers, while the cytoplasmic tail is phosphorylated by SFKs upon activation. CTLA-4’s cytoplasmic tail is highly conserved and contains two tyrosine substrates (Y201VKM and Y218FIP), which can be activated by Fyn, Lck, and potentially other kinases. These tyrosines are not typical ITSM motifs but play a role in CTLA-4’s inhibitory function by recruiting signaling molecules with SH3 domains.

Immune Checkpoint Receptor Signaling Pathways in T Cells
References:
Immune Checkpoint Receptors Signaling in T Cells. Int J Mol Sci. 2022 Apr; 23(7): 3529.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.