AM-2-19 Offers Safe and Effective Alternative to Amphotericin B
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Breakthrough in Antifungal Treatment: AM-2-19 Offers Safe and Effective Alternative to Amphotericin B
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Breakthrough in Antifungal Treatment: AM-2-19 Offers Safe and Effective Alternative to Amphotericin B
According to statistics, approximately 1.5 million people die from fungal infections worldwide each year.
Unfortunately, antifungal drugs are not only scarce (only three types), but each type faces issues such as human toxicity, increased drug resistance, or limited efficacy.
Discovered in 1955, the antifungal drug Amphotericin B (AmB) is a potent agent against fungi and is less prone to developing resistance. However, AmB has strong renal toxicity, making it the last line of defense against human fungal infections.
Recently, a research team led by Martin D. Burke from the University of Illinois Urbana-Champaign and Chad M. Rienstra from the University of Wisconsin-Madison published a groundbreaking study in the top journal “Nature.”

After more than a decade of exploration, they have found a way to improve AmB. Their modified version, AM-2-19, can kill over 500 types of fungi, demonstrating comparable or superior therapeutic effects to AmB. Importantly, even at the maximum dosage, AM-2-19 has no harmful effects on the kidneys.
Currently undergoing clinical trials, AM-2-19 could revolutionize the field of antifungal treatment if successful.
As mentioned earlier, AmB has been in clinical use as an antifungal drug since 1959.
Despite its long history, scientists have not fully understood AmB’s mechanism of action for almost half a century. The mainstream view suggests that after penetrating the fungal cell wall, AmB binds with ergosterol in the fungal cell membrane, forming pores that lead to substance leakage and fungal cell death.
However, Burke’s team, in a 2012 study, challenged this view, indicating that the holes in the cell membrane were not crucial to AmB’s fungicidal process; instead, the crucial factor was AmB’s binding with ergosterol.
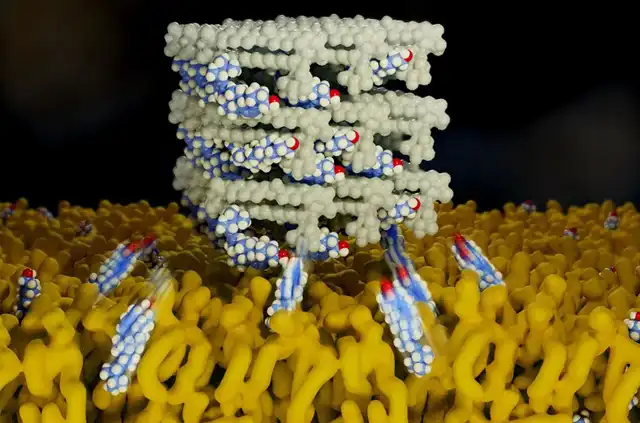
Schematic diagram of AmB removing ergosterol and cholesterol from cell membranes
Over the following years, Burke’s team collaborated with Rienstra’s team to refine their AmB killing model. Their research indicated that AmB acts like a magnet, pulling ergosterol out of the fungal cell membrane, causing changes in membrane permeability and promoting fungal death.
Simultaneously, the mechanism of AmB’s renal toxicity emerged. Similar to ergosterol in the fungal cell membrane, AmB also extracts cholesterol from human cell membranes.
After discovering this secret, Burke’s team attempted to modify AmB, creating various derivatives, but none were both safe and effective.
When Burke’s team faced a dead end, Rienstra’s team brought good news. As experts in solid-state nuclear magnetic resonance, Rienstra and his team obtained high-resolution images of AmB binding with ergosterol and cholesterol.
The atomic-level resolution model allowed the researchers to see the true nature of AmB’s binding with ergosterol and cholesterol. It turned out that the binding conformation with ergosterol and cholesterol was highly similar, explaining the previous failures. The good news was that AmB had a higher affinity for ergosterol than cholesterol. This meant there was still hope to prevent AmB from binding to cholesterol.
After in-depth analysis of imaging data, they found that swapping one hydrogen atom and one hydroxyl group in AmB (C2′epiAmB) could prevent AmB from binding to cholesterol. Experimental results showed that this new compound no longer bound to cholesterol, while retaining its ability to bind with ergosterol. Even at the highest dosage, it had no detrimental effects on the kidneys.
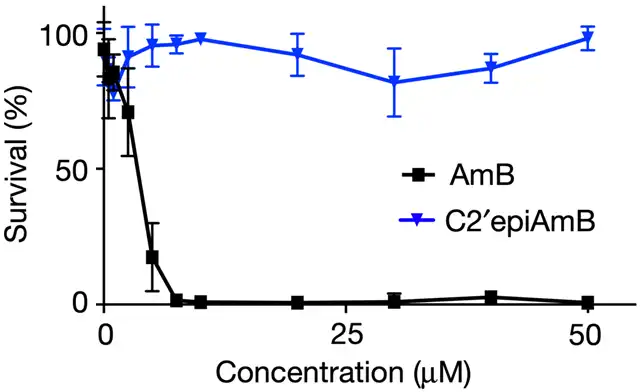
New compound (C2′epiAmB) does not cause kidney cell death
Unfortunately, success did not come quickly.
Burke and Rienstra’s team found that AmB’s binding ability with ergosterol also weakened. This weakening would slow down the extraction of ergosterol from the fungal cell membrane. For fungi that synthesize ergosterol quickly, AmB’s antifungal ability would decrease.
To prevent their previous work from going to waste, Burke and Rienstra’s team attempted to enhance the speed of AmB extracting ergosterol on the current basis. This way, they could disrupt the balance of ergosterol in the fungal cell membrane, achieving the effect of destroying the cell membrane.
So, they went back to analyzing high-resolution images.
Their efforts paid off. They found that by adjusting one carboxylic acid group, they could speed up the extraction of ergosterol by AmB derivatives. Thus, Am-2-19 was born.
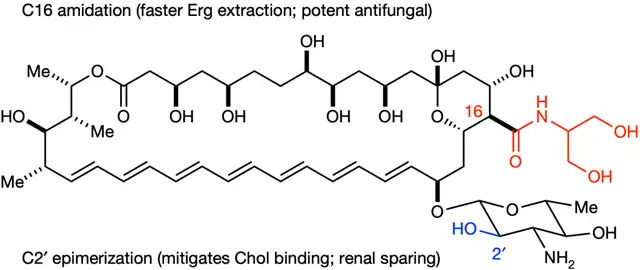
Am-2-19 himself
Experimental data showed that AM-2-19 could bind with ergosterol but not with cholesterol. It could extract ergosterol from the fungal cell membrane more rapidly, killing the fungi. Moreover, AM-2-19 was non-toxic to primary human kidney cells and other mammalian cells, causing no damage to mouse kidneys.
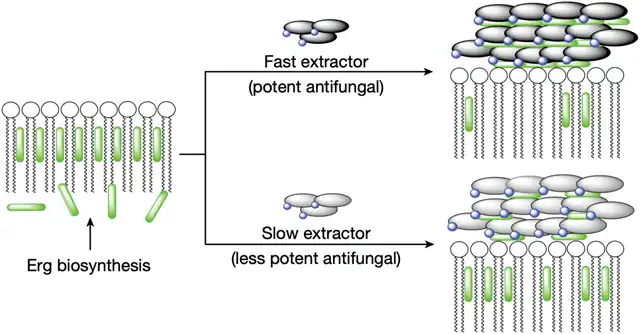
Fast extraction speed can destroy the fungal cell membrane
After obtaining these research results, Burke and Rienstra’s team sent AM-2-19 to four other laboratories to test its antifungal effects.
The experimental results showed that AM-2-19 exhibited antifungal activity against over 500 pathogenic fungi. The efficacy and spectrum of AM-2-19 were overall comparable or superior to liposomal amphotericin B (AmBisome). Importantly, AM-2-19 showed activity against several strains resistant to AmBisome.
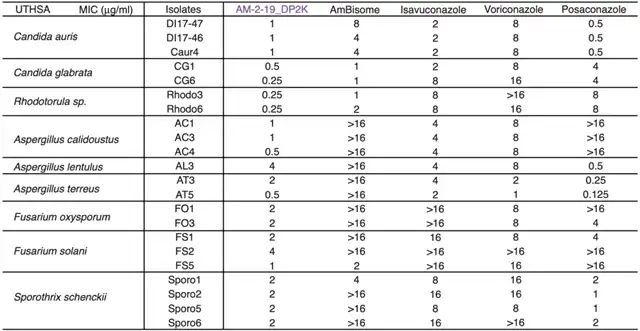
Comparison of effects in the laboratory
In summary, after more than a decade of effort, Burke and Rienstra’s team has finally decoupled the efficacy and toxicity of AmB, upgrading the last line of defense against human fungal infections to be more effective and safer.
In the future, the success of related clinical trials will undoubtedly save more lives of those infected.
Breakthrough in Antifungal Treatment: AM-2-19 Offers Safe and Effective Alternative to Amphotericin B
References:
[1].https://www.science.org/content/article/new-antifungal-kills-without-toxic-side-effects
[2].https://medicine.illinois.edu/news/new-antifungal-molecule-kills-fungi-without-toxicity-in-human-cells-mice
[3].Maji A, Soutar CP, Zhang J, et al. Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal. Nature. doi:10.1038/s41586-023-06710-4
[4].Cavassin FB, Baú-Carneiro JL, Vilas-Boas RR, Queiroz-Telles F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect Dis Ther. 2021;10(1):115-147. doi:10.1007/s40121-020-00382-7
[5].Gray KC, Palacios DS, Dailey I, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc Natl Acad Sci U S A. 2012;109(7):2234-2239. doi:10.1073/pnas.1117280109
[6].Anderson TM, Clay MC, Cioffi AG, et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol. 2014;10(5):400-406. doi:10.1038/nchembio.1496
[7].Lewandowska A, Soutar CP, Greenwood AI, et al. Fungicidal amphotericin B sponges are assemblies of staggered asymmetric homodimers encasing large void volumes. Nat Struct Mol Biol. 2021;28(12):972-981. doi:10.1038/s41594-021-00685-4
[8].Guo X, Zhang J, Li X, et al. Sterol Sponge Mechanism Is Conserved for Glycosylated Polyene Macrolides. ACS Cent Sci. 2021;7(5):781-791. doi:10.1021/acscentsci.1c00148
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



