Develop a safe and effective COVID-19 vaccine based on past experience
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Develop a safe and effective COVID-19 vaccine based on past experience
Develop a safe and effective COVID-19 vaccine based on past experience. The rapid spread of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused the same rapid response to the development of a COVID-19 vaccine.
These efforts are encouraging; however, comprehensive efficacy and safety assessments are critical to vaccine development, and we can learn from previous vaccine development activities.
This article analyzes and summarizes examples of vaccine-related disease enhancement in the history of the development of vaccines against respiratory syncytial virus, dengue virus, SARS and MERS. These examples highlight the importance of a robust safety and effectiveness profile, as well as the current Preclinical and clinical evaluation of COVID-19 vaccine candidates, vaccine design and optimization are provided.
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly around the world. The intensity and high speed of the spread of SARS-CoV-2 have resulted in high morbidity and high mortality, and has put tremendous pressure on public health systems and the global economy around the world. Therefore, the development of vaccines and treatments against COVID-19 is a top priority. Vaccines can prevent diseases in a large number of people at a relatively low cost, so they are a powerful tool to reduce the impact of COVID-19.
On March 16, 2020, Moderna’s mRNA COVID-19 vaccine (mRNA 1273) and Cansino’s non-replicating adenovirus type 5 (Ad5) COVID-19 vaccine entered phase I clinical trials. In April 2020, the inactivated COVID-19 vaccine (Sinopharm-Wuhan inactivated vaccine) and Inovio’s DNA vaccine produced by Beijing Kexing (PiCoVacc), Beijing Institute of Biological Products (BBIBP-CorV) and Wuhan Institute of Biological Products (Sinopharm-Wuhan Inactivated Vaccine) (INO-4800), entered the phase I clinical trial.
One month later, five more vaccine candidates have also entered phase I clinical trials, and more than 100 COVID-19 vaccine candidates are in laboratory or preclinical studies. The speed of COVID-19 vaccine development is encouraging. However, we and others have raised some safety issues for COVID-19 vaccine candidates.
The high-dose mRNA-1273 vaccine can protect mice from infection by the mouse-adaptive SARS-CoV-2 challenge without enhancing the level of immunopathology.
PiCoVacc and BBIBP-CorV cause neutralizing antibodies (NAbs) in mice, rats, and non-human primates. Non-human primates in the high-dose group are completely protected from SARS-CoV-2 infection, and There is no antibody-dependent enhancement (ADE).
The chimpanzee parvovirus vector vaccine (ChAdOx1 nCoV-19) developed by the University of Oxford and the University of AstraZeneca and the DNA vaccine produced by Harvard Medical School can also effectively reduce the viral load of non-human primates caused by SARS-CoV-2 , Without enhancing immunopathology.
So far, several COVID-19 vaccine phase I/II clinical trials have been completed, including the mRNA-1273 of the Ad5 nCoV trial, the ChAdOx1 nCoV-19 and the mRNA vaccine developed by Pfizer and BioNTech (BNT162b1).
According to the reported results, all these vaccines induce antibodies against spike protein (S protein) and receptor binding domain (RBD), including antibodies that neutralize pseudotyped and live SARS-CoV-2. Some reports show that NAb titer is closely related to the concentration of IgG bound to RBD.
Recently, due to unexpected adverse reactions, AstraZeneca announced the suspension of its Phase III clinical trial of ChAdOx1 nCoV-19 vaccine, although the trial has resumed in the UK.
In addition, Russia has recently approved a non-random I/II phase of the heterologous primary immunization of recombinant AD26 and recombinant Ad5 after a non-random phase I/II study-enhanced COVID-19 vaccine used in tens of thousands of people. Vaccine safety is still a key issue in phase III clinical trials and future applications of vaccines, especially for vaccine-related immunopathology that occurs when vaccine recipients are naturally infected, as described below.
In the 1960s, scientists discovered that antiviral antiserum may cause an abnormal increase in the viral infectivity of animal viruses. This phenomenon, that viral infection can be enhanced by the associated internalization of the Fc receptor (FcR) with antibodies, is denoted as “antibody-dependent enhancement” (ADE;), and subsequently in the infection of flaviviruses and other viruses Has been widely reported. Later, it was reported that more antibody FcR-mediated effects, such as complement activation and the release of inflammatory cytokines, were involved in more serious diseases.
ADE was also observed in vaccinated animals after virus challenge with the corresponding virus. For example, cats immunized with FIPV challenged with a vaccine expressing feline infectious peritonitis virus (FIPV) S protein on a recombinant poxvirus vector died earlier than control animals. Considering that passive immunization with cat serum containing high-titer antibodies that can react with cat FIPV can also lead to faster onset of disease after FIPV challenge, vaccine-induced disease deterioration may be attributed to ADE. In addition to ADE, the immunopathological response based on type 2 T helper cells (TH 2 cells) caused by homologous virus attack after vaccination may also lead to aggravation of the disease.
Key terms in disease enhancement
ADE
Antibody-dependent enhancement (ADE) can mediate the internalization of the virus through the antibody Fc receptor, which leads to more extensive virus replication and cytokine release. In the presence of virus-specific antibodies. ADe infection has been widely reported among flaviviruses and other viruses, such as HIV and influenza virus infection.
ERD
Enhanced Respiratory Disease (ERD) describes the more severe clinical symptoms after respiratory virus infection and the infection caused by previous immunity, such as respiratory syncytial virus and influenza virus infection response. ERD usually manifests as an infiltration of monocytes around the bronchus accompanied by excessive eosinophils. ERD may be vaccinated after virus infection of homotype or heterotype serotype, natural infection or transfer of maternal passive immunity.
VADE
Varde vaccine-associated disease enhancement (VADE) partially overlaps with ADE and ERD. In contrast, for ERD, VADE only deals with vaccine-related situations, and more importantly, it is not limited to respiratory diseases. For example, a dengue virus infection with a heterotypic serotype may cause more severe dengue hemorrhagic fever in vaccinated people. This phenomenon is related to VADE, but does not include ERD. VADE can be attributed to antibody-dependent and type 2 T helper cell-dependent mechanisms.
In this view, we use the term “vaccine-related disease enhancement” (VADE; box 1) to describe the exacerbation of antibody-dependent and TH 2 cell-dependent diseases (Figure 1). We have summarized the examples of VADE in the history of vaccine development against respiratory syncytial virus (RSV), dengue fever virus (DENV), SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), each of which provides safe COVID The development of the clue-19 vaccine highlights the need for rigorous preclinical and clinical safety testing.
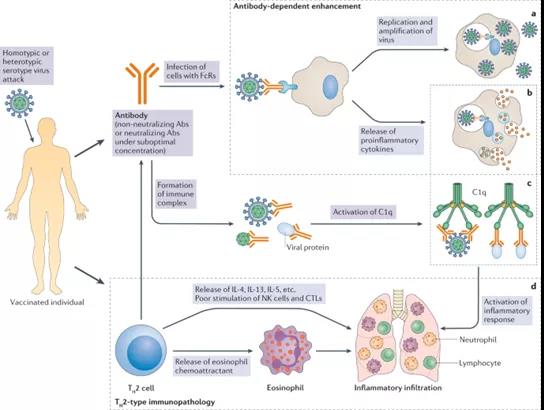
Figure 1: Mechanism of enhancement of vaccine-related diseases
Vaccination can induce humoral and cellular immune responses in immunized individuals. Under normal circumstances, when the homologous virus enters the immune body, it will be neutralized or eliminated by vaccine-induced neutralizing antibodies (Abs) or specific T cells, respectively. In the context of enhanced vaccine-related diseases, vaccines mainly induce non-neutralizing antibodies or neutralizing antibodies with low titer (suboptimal concentration) or type 2 T helper cells (TH2 cells) biased T cell responses.
When these vaccinated individuals are attacked by homotypic or heterotypic serotype viruses, antibodies will immediately recognize the virus and mediate the exacerbation of antibody-dependent diseases in two ways. First, viral antibody complexes may enter cells with Fc receptors (FcR) through FcR-mediated internalization, such as dendritic cells and monocytes, which is called “antibody-dependent enhancement” (ADE) . For viruses that are congenital to FcR-carrying cells, such as dengue virus, ADE will result in a higher viral load compared to the absence of antibodies.
After a virus enters, whether it is replicating or not, it may activate a harmful immune response and cause the release of pro-inflammatory cytokines. In addition to ADE, antibody-antigen complexes can stimulate the complement pathway through the activation of the C1q pathway, thereby further enhancing the inflammatory response. The enhancement of C1 vaccine-related diseases may also involve the T H 2 cell-biased immune response. The activated T H 2 cells contribute to the activation of antibody production. However, they release interleukin 4 (IL-4), IL-13 and IL-5, and eosinophil chemotactic factors, which lead to eosinophil infiltration and the production of pro-inflammatory cytokines in the lungs.
d | Natural killer (NK) cells and CD8 + cytotoxic T lymphocytes (CTL) are less stimulating in T H. Two kinds of cells have skewed immune responses. Excessive cytokine release (part b), activation of the complement pathway (part c), and excessive mobilization of eosinophils all contribute to the infiltration of eosinophils, neutrophils and lymphocytes into the lungs, and Lead to the production of inflammatory cytokines (part d), leading to acute lung injury or acute respiratory distress syndrome.
Lessons learned from the RSV vaccine
There have been warnings that a comprehensive assessment of the ADE of the coronavirus vaccine should be conducted to avoid repeated tragic failures of the RSV vaccine. The first RSV vaccine based on formalin-inactivated RSV (FI-RSV) entered clinical trials in 1965. At that time, several other inactivated or attenuated virus-based vaccines, such as smallpox and Vaccine for polio. The FI-RSV vaccine is well tolerated and appears to be moderately immunogenic at first.
But the FI-RSV vaccine did not protect the study participants, but showed a paradoxical disease enhancement effect (enhanced respiratory disease (ERD); box 1) during subsequent natural RSV infection. Of the 20 infants who received the FI-RSV vaccine, 16 required hospitalization, including 2 infants who died subsequently, while only one of the 21 participants in the control group was hospitalized. The FDA then urgently suspended all clinical studies of the RSV vaccine.
In order to clarify the mechanism of ERD in this RSV vaccine trial, the humoral and cellular immune responses after FI-RSV vaccination were analyzed. FI-RSV induces RSV glycoprotein binding, but not NAb, eosinophilia and exaggerated CD4 + T cell responses. It was not until the 1990s, thirty years after the first FI-RSV test, that it was discovered that the inflammatory response to the vaccine was enhanced, including the skewed T cell response of TH 2 cells, which led to the excessive proliferation of CD4 +. T cells and eosinophils. This T^h leads to poor stimulation of natural killer cells and CD8. 2 cell deflection patterns + cytotoxic T lymphocytes, which otherwise can prevent TH2 cells and inflammatory response to RSV antigen.
Recent work has shown that the carbonyl group caused by formalin fixation produces an enhanced T H 2 cell response. However, ERD was also observed in experimental animals that were not immunized with formalin-fixed purified RSV F and G glycoprotein, indicating that formalin fixation is not a determinant of pathogenic inflammation. Previous studies have shown that FI-RSV induces the main TH2 cell-like cytokine profile, such as interleukin 5 (IL-5) and IL-13, while live RSV that does not cause ERD induces the main type 1 T helper cells ( TH 1 cell)-like cytokine profile. Such as IL-10).
In addition, some live attenuated RSV vaccines and some RSV antigens expressed by viruses or DNA vectors are not induced, or only slightly induced, and ERD is in humans. One of the reasons why only certain antigens induce ERD can be the diverse structure of the surface glycoprotein RSV display, thereby inducing different immune responses. Indeed, some studies have shown that the exposed antigenic sites are different between the surface proteins before and after the fusion, and even antibodies that target the shared site may not be able to bind the two conformations equally.
It is worth noting that another study reported that as long as the antigen concentration is high and the vaccine contains a T H 1 cell-biased adjuvant, the F protein can protect the vaccinated cotton rats after and before the fusion. In addition to the skewed immune response of TH 2 cells, antibody-mediated effects can also contribute to ERD. FI-RSV binds antigen-induced non-NAb binding, and then the antibody-antigen complex stimulates the complement pathway, thereby further enhancing the inflammatory response.
In 2019, the RSV vaccine (Ad26.RSV.preF) based on the adenovirus vector expressing the RSV F protein stable in the pre-fusion conformation passed the FDA breakthrough treatment designation plan to prevent RSV in the elderly. Ad26.RSV.preF induces high-titer NAb and long-lasting TH 1 cell biased immunity, which is characterized by a high ratio of interferon-γ (TH type 1 cytokine) and TH type 2 cytokine (IL-4), IL-5 or IL-10) in adult and newborn mice.
However, clinical trials of Ad26.RSV.preF are only conducted in adults 60 years of age or older; therefore, the RSV vaccine for infants is still elusive. Therefore, in exploring the 50-year history of RSV vaccines, regardless of the current urgency, we all know the absolute necessity of tracking the overall safety of the vaccine before large-scale application. From RSV experience, although we do know that the antigen conformation and the relationship between the pre-fusion and the fusion state are important, we still do not know which characteristics of the antigen will cause the disease to worsen. We also learned that the biased immune response of TH 2 cells is harmful.
For example, antigen-induced T H 2 cell-like cytokine profiles, such as IL-5 and IL-13, can activate CD4 + T cells, but have a weak ability to stimulate natural killer cells and CD8 +. T cells in animal models or humans. The biased immune response of such TH 2 cells may cause VADE to be attacked by viruses. In addition, we learned that it is critical to induce NAbs to exceed bound antibodies.
Lessons from Dengue Vaccine
Similar to RSV, the development of dengue vaccines started with virus-based inactivated vaccines. In the 1920s, Blanc and Cminopetros inoculated study participants with a bile-DENV mixture. However, this vaccine failed to protect participants from subsequent DENV attacks. Since then, many studies have found that the high titer caused by natural dengue virus infection and the patient’s sustained NAb response to homologous DENV.
A team of researchers obtained an attenuated strain of DENV by continuously spreading DENV in the mouse brain. One dose of attenuated vaccine is sufficient to induce NAb in vaccinated volunteers. There are four serotypes of DENV (DENV1-DENV4), which have considerable similarities in epitopes. The induced NAbs can not only protect patients from homologous virus infection, but also cross-react with heterologous DENV.
However, the protection period of the latter is shorter than 3 months to 2 years. What is important is that once the cross-specific antibody drops to a suboptimal concentration after being naturally infected with heterologous DENV, the risk of causing severe dengue fever symptoms is higher than that of uninfected individuals.
This phenomenon has been extensively studied. The cross-reactive antibody binds to the heterologous DENV, thus facilitating the entry of the virus into the FcR of target cells, such as monocytes, macrophages and dendritic cells. At the same time, epidemiological studies have shown that the occurrence of severe dengue fever is associated with a range of cross-reactive antibody titers (DENV antibody titers from 1:211:80).
In addition to the enhancement of entry, non-NAb or NAb at a lower than optimal concentration may form a complex with DENV particles, and then cause an inflammatory response through the FcR-mediated immunomodulatory pathway 62, which further increases the risk of severe dengue fever.
Obviously, the re-infection of heterotypic serotype DENV caused ADE. Therefore, the next challenge for dengue vaccine development is the induction of NAb for all four DENV serotypes. It was not until 2006, 77 years after the first inactivated dengue vaccine was tested in humans, that the first quadrivalent dengue vaccine CYD-TDV entered clinical trials (NCT00384670).
CYD-TDV is a recombinant attenuated live vaccine that expresses four serotypes of DENV on the backbone of yellow fever. In 2018, the FDA approved the CYD-TDV vaccine to prevent dengue fever caused by all serotypes (DENV1-DENV4). However, individuals who have not previously been infected with DENV are not allowed to receive the vaccine. This decision was made because clinical analysis has shown that people who have been vaccinated negative have a greater risk of dengue fever than people who have not been vaccinated negative.
Since DENV can infect cells with FcR, but SARS-CoV-2 cannot, the ADE of dengue virus infection and disease may be more significant than that of COVID-19. In COVID-19, the ADE of virus infection and disease may be more significant. Light or not even. In addition, the pathophysiology of dengue fever cannot be compared with COVID-19. Therefore, the VADE mechanism of DENV may have nothing to do with the mechanism in SARS-CoV-2. Nevertheless, valuable experience can be learned from the difficult and arduous task of developing a dengue vaccine.
First of all, in addition to neutralizing activity, we know that any vaccine-induced antibody titer should be fully evaluated. It has been observed in both DENV infection 58 and RSV infection 50 that low titers of NAbs can cause subsequent ADE infections, rather than provide protection. Secondly, the population genetic analysis of 103 SARS-CoV-2 genomes showed that based on the different gene mutations in ORF1ab and ORF8, SARS-CoV-2 has evolved into two main types (L and S) (Reference).
Further research found that during the early evolution of SARS-CoV-2, there were 382 nucleotide deletions in ORF8). In the S pandemic, the SARS-CoV-2 variant with the D614G change in the S protein became the most common. More than six human coronaviruses are prevalent in human populations, and more prevalent in wild animal species. So far, it is not clear whether the continued mutation and recombination of SARS-CoV-2 will produce other serotypes of SARS-CoV-2, or even other new coronaviruses.
Therefore, candidate vaccines that can provide protection from different coronaviruses would be ideal. Third, clinical data from a large sample shows that the performance and efficacy of dengue vaccine can be affected by serotype, baseline serum status and age. These results indicate that COVID-19 candidate vaccines should be fully evaluated in multiple animal models (ie, adult and adult animals and male and female animals) to confirm their safety and effectiveness, while human research participants should reflect different Crowd.
Depending on age and gender, the different severity of COVID-19 further highlights this point. Older and male individuals are at higher risk of developing serious diseases during the initial infection.
Lessons learned from SARS and MERS vaccines
The genomes of SARS-CoV-2 and SARS-CoV have 79.6% sequence identity70, and they use the same receptor angiotensin-converting enzyme 2 (ACE2) to enter cells. Therefore, the immune response induced by the SARS vaccine that has been studied will be useful for the evaluation of COVID-19 vaccine candidates. In 2003, after the SARS-CoV virus particles were isolated and the virus genome sequence was released, the SARS vaccine design began.
Similar to COVID-19 vaccine developers, researchers first sought a SARS vaccine based on inactivated viruses, recombinant subunit proteins and recombinant vectors. Also in 2003, an Ad5 vector-based vaccine expressing SARS-CoVS1 protein, membrane (M) protein and nucleocapsid (N) protein was tested in rhesus monkeys. These vaccines induced SARS-CoV specific T cell and NAb responses. Ad5-SARS-CoV-S caused a significant reduction in viral load and prevented severe pneumonia in ferrets.
The recombinant modified vaccinia virus Ankara vector expressing SARS-CoVS protein caused a rapid and powerful NAb response in ferrets. However, a strong inflammatory response in the liver of immunized ferrets appeared after the SARS coronavirus attack. More studies have shown that the SARS vaccine, based on the inactivated virus or recombinant vector, can induce eosinophils and T H in a mouse model with SARS-CoV’s subsequent attack 2 cell skewed immune response, which makes people Think of infant ERD induced by RSV vaccine.
Similarly, inactivated SARS-CoV vaccine and SARS-CoVS protein-derived peptide vaccines will induce more severe lung damage in rhesus monkeys after SARS-CoV challenge. The DNA vaccine encoding the S protein of the SARS-CoV virus induces CD4 + and CD8 + T cell and NAB responses in a mouse model and is undergoing phase I clinical trials.
ADE has also been observed in SARS vaccines. The SARS vaccine based on recombinant SARS-CoVS protein can protect hamsters from SARS-CoV infection; however, S protein-specific antibodies can mediate FcR-dependent entry into B cells in vitro. In addition, diluted SARS-CoVS protein-specific antibodies lead to enhanced viral infectivity and cytopathic effects in the HL-CZ human primary cell line.
According to reports, in addition to ADE, antibody-mediated imbalanced macrophage activation is associated with significant lung injury in vivo. Passive transfer of anti-SIgG eliminates the wound healing response and promotes the recruitment and accumulation of pro-inflammatory monocytes and macrophages in the lungs of rhesus monkeys after virus attack, which indicates that SARS-CoVS protein specific antibodies can cause pathogenic immune responses , And enhance the severity of the disease after SARS coronavirus infection.
It is worth noting that evidence of anti-SIgG-mediated ADE was only observed in vitro. Therefore, compared with other antibodies and TH2 cell-mediated immunopathology in the body, ADE does not seem to be that critical issue.
MERS-CoV belongs to the Betacoronavirus genus, which also includes SARS-CoV and SARS-CoV-2. Since the virus was first discovered in Saudi Arabia in 2012, many vaccine technologies have been developed, including subunit vaccines, viral vectors and DNA-based vaccines, inactivated and live attenuated vaccines, and the development of MERS vaccines. Many of them can induce an adequate immune response and protect the vaccinated animals from subsequent MERS-CoV infection. However, two studies reported that the mice independently vaccinated with the inactivated MERS-CoV vaccine developed a 2-cell biased immune response and increased eosinophil infiltration after virus challenge.
Several pieces of evidence indicate that MERSS protein-specific antibodies can mediate ADE. The monoclonal antibody induced by recombinant MERS-CoVS1 binds to the cell surface IgG FcR and mediates the entry of the virus into HEK293T cells and macrophages expressing FcR exogenously (induced by THP-1 monocytes) through a regulated virus receptor dependence The pathway endogenously expresses FcRs. Rabbits infected with MERS-CoV produced MERS-CoVS protein-specific antibodies, without neutralizing activity and protection from reinfection. Therefore, rabbits infected with MERS-CoV showed pulmonary inflammation related to complement activation. Enhanced.
Overall, the VADE signs of the MERS vaccine are not as prominent as the SARS vaccine. At present, a DNAMERS vaccine (INO-4700) and two protein-based vaccines based on the virus-carrier MERS have shown good safety and phase I clinical trials of induced humoral and cellular immune responses against MERS-CoV . The aforementioned VADE phenomenon in the development of SARS and MERS vaccines further highlights the lessons we have learned from RSV and DENV. First, a vaccine candidate for SARS-CoV-2 should induce a balanced T cell response.
In particular, the immune response of cells should be evaluated in animals and humans after TH 1 and TH2 vaccination. Second, only diluted SARS-CoVS protein-specific antibodies can increase the infectivity of the virus, indicating that VADE is related to the antibody titer of immunized subjects.
VADE’s presumption mechanism
At present, the basic mechanism of VADE is not clear, because its emergence is highly virus, host and antigen specific. However, vaccines have some common features and can induce VADE in vivo. First, vaccines used to target and replicate in FcR-bearing cells infected with viruses (including DENV and Ebola) are likely to induce VADE, especially ADE. So far, only one study has reported that monocytes and B and T lymphocytes are susceptible to SARS-CoV-2 active infection, and the report has not been peer-reviewed.
Therefore, more efforts are needed to alleviate this concern. Secondly, the virus infection vaccine used in the vaccine can cause inflammatory damage, which is likely to cause VADE. For example, SARS-CoV and RSV. About 13.9% of COVID-19 patients develop severe pneumonia, in which inflammation is the cause of the pathology. Preliminary reports show that in randomized trials, the 28-day mortality rate of the COVID-19 patient group who received dexamethasone, which has anti-inflammatory effects and conventional treatment, was lower than that of patients who received conventional treatment alone. However, pathology seems to be highly dependent on the host.
Therefore, there are no definite markers that can predict which patient will develop acute respiratory distress syndrome. Similarly, it is still difficult to predict which antigen will cause VADE. Third, antigens that cause non-neutralizing antibodies or insufficient antigens are likely to cause VADE. Several series of evidence indicate that RBD-specific IgG and NAb are in patients with COVID-19.
However, the duration of the antibody response and the potential for long-term protection against subsequent natural infections are unknown. The reported antibody response kinetics to SARS-CoV-2 infection are different. For example, one study reported that “severe infections were associated with early seroconversion”, while another reported “delayed, but stronger antibody responses were observed in critically ill patients”. In addition, the last two cases with SARS-COV-2, in the case of reinfection in the United States and Ecuador, showed severe symptoms in the second round of infection, and 2 reinfection cases in Hong Kong and Europe showed more symptoms in the second round. light.
It is worth noting that the first round of infection did not cause seroconversion in Hong Kong patients, which may be the most critical determinant of the second round of infection. In short, we still do not fully understand the antibody dynamics of COVID-19 patients, which is why we need to carefully evaluate the immune response of candidate vaccines in animal models and clinical trials, which will be discussed below.
Impact on the COVID-19 vaccine: Animal models used to evaluate the safety and effectiveness of COVID-19 vaccines
Vaccines should be very effective in eliciting humoral and cellular responses in the body, because low titers of NAb and insufficient activation of CD8 + T cells 12 are risk factors for VADE. At the same time, we see two major obstacles to safety assessment. First, it usually takes a long time to observe VADE, because it mainly appears in subsequent attacks or natural infections, caused by homologous or heterologous virus strains, and this occurrence is usually related to the reduction of antibody titers to sub-optimal levels .
Second, it is not clear whether laboratory animals can accurately represent human responses. From the past experience and lessons in the development of RSV, Dengue fever, SARS and MERS vaccines, we provide the following suggestions for developers of safe and effective COVID-19 vaccines.
First, the safety of candidate COVID-19 vaccines should be evaluated in multiple animal models. Since there is no animal model that can accurately simulate the human immune response to candidate vaccines, evaluation in several animal models can avoid the risk of missing pathogenic responses. Secondly, the attack of heterogeneous virus strains should be used in COVID-19 vaccine evaluation, and the antibody should cross-react with SARS-CoV and SARS-CoV-2. Third, the experiment should be repeated in the same animal model at different ages.
Previous studies have proved that the performance and efficacy of dengue vaccines can be affected by serotype, baseline serum status and age. The immunopathology of TH2 cell preference was mainly observed in aging mice immunized with inactivated SARS-CoV and the alum adjuvant 76. Venezuelan equine encephalitis virus replicon particles expressing SARS-CoVS protein provide complete short-term protection against heterologous SARS-CoV challenge in young mice, but have limited protection in vaccinated aging animals. Given that the elderly are the most susceptible to COVID-19, it is important to evaluate safety and efficacy in aging animal models and humans.
Fourth, considering that the clinical results of patients with COVID-19 comorbidities are worse than those without comorbidities, and the incidence of comorbidities is related to comorbidities, animal experiments should also be carried out in animal models of comorbidities and humans. And poor clinical results from clinical trials.
Parameters to assess the safety and effectiveness of the COVID-19 vaccine
Previously, some people have proposed some parameters that are indispensable in the evaluation of the safety and effectiveness of coronavirus vaccines, including the geometric mean titer of NAbs, the ratio of NAb titer to non-neutralizing antibody titer, antibody affinity, and T cell response. Spectrum, virus titer. The upper respiratory tract and the lower respiratory tract, and use immunohistochemistry to identify viral antigens and immune cell markers in lung histopathology.
The vaccine-induced NAbs titer is the most important indicator of effectiveness and safety evaluation, because the suboptimal concentration of NAbs cannot effectively neutralize and may enhance SARS-CoV-2 infection. Moore and Klasse concluded in a review: “It is not clear what benchmark serum antibody and NAb titers must be achieved for the SARS-CoV-2S protein vaccine to protect humans. The animal attack experiments reviewed above indicate that immunity should be sterilized. , A serum NAbID 50 titer in the range of about 100 to 500 is required.” We also noticed that there was no detectable SARS-CoVRNA in the lung tissue of vaccinated mice with a serum NAb titer of 1:189 or higher. . The FDA recommends that the plasma NAb titer during the recovery period of passive therapy is at least 1:160.
Therefore, we suggest that an effective and safe COVID-19 vaccine should be able to induce antiserum against SARS-CoV-2 in vivo infection in a mouse model with a neutralizing titer of at least 1:160. Enhanced eosinophil filtration in the lungs is one of the strongest indicators of VADE to cause SARS vaccine or MERS vaccine. When one is attacked by a virus or evaluates the safety of the COVID-19 vaccine, it should also be monitored for natural virus infection. According to the report of Chen et al. The eosinophil content in the lungs of mice immunized with the safe SARS vaccine should be less than 5% of the infiltrated cells after the virus challenge.
Therefore, we suggest that eosinophil infiltration in the lungs of inoculated mice after virus challenge should reach 5% or higher as a hypothetical parameter of VADE. How long the vaccine-induced NAb response can last is another parameter to evaluate the safety and effectiveness of the vaccine. Seow et al. Recent reports indicate that NAb titers with lower titers in some convalescent patients fall to undetectable levels within 2-3 months, indicating that NAb may not last for a long time. In contrast, a large-scale study in Iceland showed that antiviral antibodies against SARS-CoV-2 can last for at least 4 months.
Another study found that SARS-CoV-2S protein-specific memory B cells and circulating follicular helper T cells are positively correlated with plasma neutralization activity. Therefore, these two indicators can be used to monitor the lifespan of the immune response to SARS-CoV-2 after vaccination. Our previous studies have shown that NAb in the serum of mice immunized with the RBD-based SARS vaccine can maintain a high titer (1:580) for 6 months. Therefore, we recommend that in vaccinated mice, the NAb response caused by the COVID-19 vaccine should last at least 6 months.
The best antigen for designing a safe and effective COVID-19 vaccine
The ideal antigen should be selected to develop a safe and effective COVID-19 vaccine. The S protein is the main antigen in most of the COVID-19 vaccine candidates being developed because it contains the main neutralizing epitope and is located on the surface of the virus particle. However, the full-length S protein of SARS coronavirus also contains several immunodominant sites that can induce non-neutralizing antibodies, including those associated with ADE, or harmful immune responses. For example, compared with antibodies from non-immunized rhesus monkeys, antibodies against the S597-603 epitope (located near the carboxyl end of the RBD of the SARS-CoVS protein) significantly enhanced the SARS-CoV infection of VeroE6 cells.
The RBD subunit of the S protein of SARS coronavirus caused a strong NAb response and protection challenge against SARS-CoV, without obvious VADE, in the mouse model. Our previous studies have shown that RBD contains a higher titre of NAb that can be induced by the main neutralizing epitope of S protein, but a lower level of non-neutralizing antibodies, compared with S1 subunit or full-length S protein. SARS-CoVRBD and Alhydrogel (1:25) as adjuvants induced strong protection without signs of VADE, while the full-length SARS-CoVS protein induced weak protection and strong VADE in mouse model 115. At the same time, most of the NAbs isolated from the serum of coronavirus-infected patients target RBD.
In addition, SARS-CoV-2RBD elicited an effective neutralization response without ADE in mice 130. RBD-dimer vaccines against COVID-19, SARS or MERS induced NAb responses to the corresponding viruses and showed high yields in pilot-scale production. Our recent studies have shown that lipid nanoparticle-embedded RBD-based mRNA COVID-19 vaccine can trigger a strong T cell response and high-efficiency NAb against SARS-CoV-2 live infections after boosting immunization within 70 days.
The NAb titer is 1:540. Mice. These antibodies can also cross-neutralize the SARS-CoV pseudovirus expressing the human SARS-CoV strains Tor2 and GD03 and the A protein of the palm civet cat strain SZ3, which indicates that this RBD-based mRNA vaccine may be further developed as safe. Effective vaccine. Effective vaccine to prevent SARS-CoV-2 and SARS-CoV infection.
Another lipid nanoparticle-encapsulated SARS-CoV-2RBD-based mRNA vaccine (ARCoV) triggered a powerful NAb and TH1 cell response in favor of mice and non-human primates, while in the previous model 133 The medium confers complete protection against the SARS-CoV-2 challenge adapted to mice.
In addition, some research groups have reported the identification of RBD targeting antibodies and cross-reactive antibodies against SARS-CoV and other human coronaviruses, indicating that there may be some conserved epitopes in RBD. A study identified eight RBD-targeting antibodies derived from SERS patients. These antibodies neutralized the real SARS-CoV-2, SARS-CoV and WIV1 coronaviruses with a maximum inhibitory concentration of half, respectively 0.05–1.4 , 0.004–0.06 and 0.076–1.7μgml -1,. Another study isolated the RBD-specific antibody S309 from the memory B cells of SARS patients. It effectively neutralized SARS-CoV-2 and SARS-CoV infections.
RBD from human SARS-CoV strain (GD03) and palm civet strain (SZ16) caused antibodies that strongly reacted with and neutralized SARS-CoV and SARS-CoV-2 in rabbits, indicating that RBD can induce cross-targeting against SARS-CoV And SARS-CoV-2 neutralizing antibody. These studies further support the development of RBD-based vaccines. Optimization of RBD by covering non-neutralizing antibody epitopes with glycosylation and exposing NAb epitopes through deglycosylation is expected to enhance its protective immunity and reduce its potential to induce non-neutralizing antibodies, which indicates that optimized RBD is a development The ideal antigen for a safe and effective COVID-19 vaccine, although other methods may also prove to be safe and effective.
Conclusion and prospects
In May 1796, a young boy was vaccinated with festering serous from a patient infected with vaccinia, thus starting the history of vaccination. Since then, vaccines have been fighting against many viral diseases such as smallpox, rabies and polio.
However, the VADE phenomenon has created substantial obstacles to the development of vaccines for certain viruses (including RSV, DENV, SARS-CoV and MERS-CoV). At present, the continued spread of COVID-19 has prompted some countries to rush to approve local vaccines without a comprehensive safety assessment.
Vaccines with viruses with high infectivity but low fatality rate (such as SARS-CoV-2) should generally have higher safety standards than viruses with low infectivity but high fatality rates (such as Ebola virus), because more Healthy individuals will have to use them.
On July 15, 2020, the World Health Organization announced that more than 150 countries have participated in the COVID-19 Vaccine Global Access Program (COVAX), a mechanism designed to ensure rapid, fair and equitable access to COVID-19 vaccines worldwide.
This further raises the safety standards of the COVID-19 vaccine, because it should be safe for everyone in the world, regardless of age, gender, race, or complications. If the adverse reaction rate of the COVID-19 vaccine is only 1%, then the world’s population will be vaccinated, and approximately 78 million people will be affected.
If the COVID-19 vaccine is distributed globally, its adverse reaction rate should be kept at a very low level. Comprehensive safety assessment and rational design of antigens and adjuvants in different animal models and clinical trials will help reduce the incidence of VADE.
(source:internet, reference only)
Disclaimer of medicaltrend.org



