Contrasting Immune Effects of Breast Cancer Chemotherapy: EC vs. Docetaxel
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Contrasting Immune Effects of Breast Cancer Chemotherapy: EC vs. Docetaxel
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Contrasting Immune Effects of Breast Cancer Chemotherapy: EC vs. Docetaxel
The commonly used EC regimen in chemotherapy for breast cancer has immunosuppressive effects, while docetaxel promotes immune activity.
Chemotherapy induces immunogenic cell death in tumor cells, promotes the release of immune regulatory molecules and tumor antigens, making the combination of chemotherapy and immunotherapy promising. Simultaneously, the potential detrimental effects of chemotherapy on immune cells, even exerting opposite effects when combined with immunotherapy, have become a new area of consideration.
Researchers, led by Rudolf Oehler at the Medical University of Vienna, have shed light on this matter. Analyzing data from 154 breast cancer patients in the ABCSG-34 clinical trial, they found that the commonly used EC regimen (epirubicin and cyclophosphamide) in breast cancer chemotherapy significantly suppresses the immune system, leading to a reduction in lymphocytes.
Conversely, docetaxel stimulates tumor cells to release more immune regulatory factors, enhancing immune activity.
The findings were published recently in the Journal of Experimental & Clinical Cancer Research [1].

ABCSG-34 is a randomized phase II clinical trial for HER2-negative early-stage primary breast cancer patients. In this trial, patients were randomly assigned to two groups receiving two different sequences of neoadjuvant chemotherapy.
Conventional sequence: epirubicin and cyclophosphamide (EC) followed by docetaxel (D).
Reverse sequence: docetaxel followed by EC.
Tumor tissue and blood samples were collected at baseline, mid-treatment, and surgery.
The unique design of the ABCSG-34 clinical trial provided an ideal platform to investigate the impact of EC and docetaxel chemotherapy regimens on the immune system.
In their analysis of data from 154 patients in the ABCSG-34 trial, Oehler and his team aimed to explore the specific effects of EC and docetaxel on immune cells.
Results showed that regardless of treatment response, EC treatment exhibited significant immunosuppressive effects. After four weeks of EC treatment, the lymphocyte count in the peripheral blood of breast cancer patients significantly decreased from 1.57×103 ±0.52 cells/μl to 0.86×103 ±0.42 cells/μl (p <0.001).
In particular, B cells experienced a strong and persistent inhibition by EC treatment, leading to a remarkable 94% reduction in B cell numbers. This inhibitory effect persisted even after four weeks of docetaxel treatment, and vice versa. The impact of EC treatment on T cells was less pronounced and more transient, resulting in a 45% reduction.
Additionally, EC treatment was found to downregulate soluble immune regulatory factors such as uPA, CXCL10, sTNFSF10, suPAR, and CCL8 in breast cancer patients. However, EC treatment did not affect the function of cytotoxic T cells.
In contrast, docetaxel treatment not only maintained lymphocyte count and function but also significantly increased the levels of soluble immune regulatory factors.
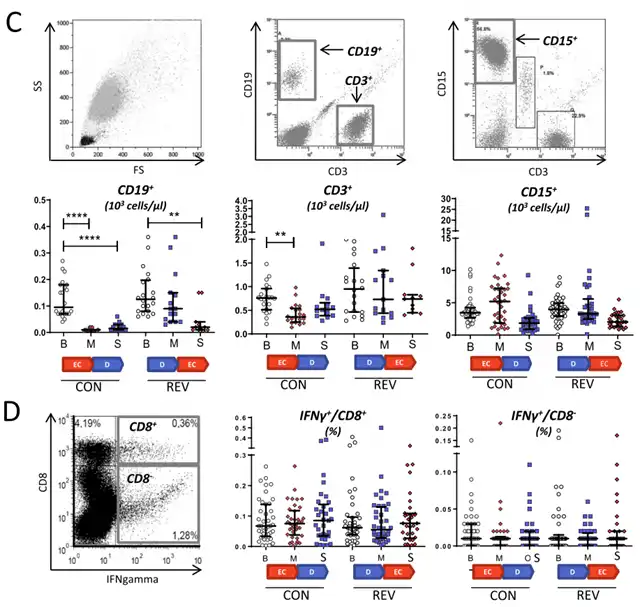
Effects on immune cell number and function
Subsequent in vitro experiments showed that the increased immune regulatory factors after docetaxel treatment mainly originated from dead tumor cells.
The researchers also compared the toxic effects of different chemotherapy drugs. Results revealed that docetaxel induced tumor cell expansion, forming giant cells with multiple nuclei and accumulating tumor cells in the G2 phase. Epirubicin-induced tumor cells exhibited typical apoptosis features, including cell shrinkage, abundant extracellular vesicle release, higher activation levels of apoptosis factor caspase-3, and an increase in G1 phase tumor cells.
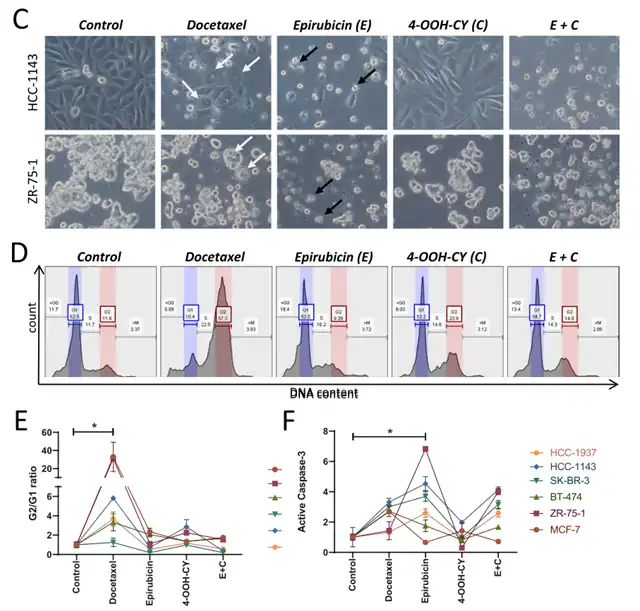 Toxic effects on breast cancer cells
Toxic effects on breast cancer cells
Previous results from the ABCSG-34 clinical trial showed no significant differences in residual cancer burden (40.1% vs. 37.2%; P =0.61) and pathological complete response rates (24.3% vs. 25%, P=0.89) between breast cancer patients receiving the sequence of EC followed by docetaxel and those receiving docetaxel followed by EC [2]. In this study, Oehler and his team shifted their perspective and discovered different impacts of these two regimens on the immune system.
The researchers suggest that the strong inhibitory effect of EC treatment on lymphocytes may counteract the stimulating effect of immunotherapy on the immune system. When planning future combination therapies for breast cancer immunotherapy, the distinct immune regulatory effects of EC and docetaxel treatments should be taken into consideration.
Contrasting Immune Effects of Breast Cancer Chemotherapy: EC vs. Docetaxel
Reference:
[1] https://jeccr.biomedcentral.com/articles/10.1186/s13046-023-02876
[2] Bartsch R, Singer CF, Pfeiler G, Hubalek M, Stoeger H, Pichler A, Petru E, Bjelic-Radisic V, Greil R, Rudas M, Muy-Kheng TM, Wette V, Petzer AL, Sevelda P, Egle D, Dubsky PC, Filipits M, Fitzal F, Exner R, Jakesz R, Balic M, Tinchon C, Bago-Horvath Z, Frantal S, Gnant M; Austrian Breast and Colorectal Cancer Study Group. Conventional versus reverse sequence of neoadjuvant epirubicin/cyclophosphamide and docetaxel: sequencing results from ABCSG-34. Br J Cancer. 2021 May;124(11):1795-1802. doi: 10.1038/s41416-021-01284-2.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



