First CRISPR Gene Editing Therapy Will Revolutionize the Pharmaceutical Industry?
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
First CRISPR Gene Editing Therapy Will Revolutionize the Pharmaceutical Industry?
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
First CRISPR Gene Editing Therapy Will Revolutionize the Pharmaceutical Industry?
First CRISPR Gene Editing Therapy to Receive FDA Approval Next Month – Will It Revolutionize the Pharmaceutical Industry?
The world’s first CRISPR gene editing therapy is poised to receive FDA approval next month, potentially opening a new chapter and disrupting the entire medical field while presenting new investment opportunities.
According to reports on Saturday, the CRISPR gene editing therapy, examglogene autotemcel (abbreviated as Exa-cel, brand name Casgevy), developed in collaboration between the U.S.-based Vertex Pharmaceuticals and Switzerland’s CRISPR Therapeutics, may obtain approval from the U.S. Food and Drug Administration (FDA) early next month.
This therapy is intended for patients with sickle cell anemia.
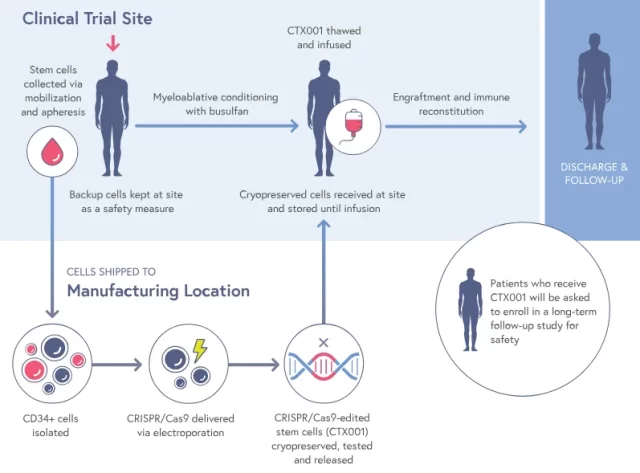
It’s worth noting that this is the world’s first approved application of CRISPR, the “gene scissors” technology. CRISPR gene editing technology, likened to molecular scissors, can be used to cut and modify DNA sequences.
Earlier this month, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) conditionally granted approval for this therapy to be marketed, targeting patients aged 12 and above with sickle cell anemia (SCD) accompanied by recurrent vaso-occlusive crises (VOCs), as well as transfusion-dependent beta-thalassemia (TDT) patients unable to undergo human leukocyte antigen (HLA)-matched hematopoietic stem cell transplantation.
With advancements in gene editing technology in human research, these regulatory approvals may only mark the beginning. Nobel Prize laureate Jennifer Doudna commented in an interview:
“This approval is a turning point in the medical field, demonstrating how genome editing can be used as a one-time treatment for diseases.”
However, this emerging field faces numerous challenges, including various safety factors and the substantial cost in the range of “millions of dollars.”
Unlimited Potential of Gene Editing
Gene editing is part of a broader revolution in therapies, including gene and cell medicine. Unlike conventional pills and injections that target proteins or pathways within the body, gene and cell therapies can address the root cause of diseases and potentially achieve cures.
The rapid development of CRISPR gene editing technology is attributed, in part, to its relative ease of use. Doudna explained that once discovered, people worldwide began applying it to their areas of interest.
She also highlighted the timeliness of this technology:
“We had a lot of information about genes; we had the ability to synthesize genes and even fragments of entire genomes. What we didn’t have at that time was a tool to rewrite the genome, and CRISPR is providing that tool, almost like a missing piece in the entire ecosystem.”
Looking ahead, for gene editing to expand its therapeutic potential, it must find ways to extend to tissues and organs. Doudna mentioned exciting long-term development areas like cancer and Alzheimer’s disease. For example, using CRISPR to alter genes that may lead to susceptibility to Alzheimer’s could protect those genetically predisposed to the disease, representing a highly promising direction.
According to statistics from Allied Market Research and Global Market Research, the global gene editing industry market size grew from $4.811 billion in 2021 to $5.412 billion in 2022, with a year-on-year growth rate of 12.49%. It is projected that by 2030, the global gene editing market size will reach $36.061 billion, with a compound annual growth rate of 22.3%.
Safety and High Costs as Application Challenges
However, the road ahead is filled with challenges, with safety and cost being the foremost considerations. Typically, the first drugs to market are not necessarily the best or most profitable.
For the Vertex/CRISPR therapy, the process is referred to as ex vivo therapy, meaning cells need to be collected from the patient, transported to a production facility, undergo gene processing using CRISPR in the laboratory, and then be returned to the hospital.
Importantly, patients need to undergo chemotherapy before cell infusion, carrying the risk of infertility. Additionally, Casgevy may face competition, as later in December, the U.S. Food and Drug Administration may approve a gene therapy for sickle cell patients provided by Bluebird Bio.
Daniel Lyons, portfolio manager at Janus Henderson, remarked:
“From a medical and scientific standpoint, this is incredibly exciting. However, when you consider how many patients are willing to undergo essentially a stem cell transplant to get this treatment, you have to be cautious.”
Furthermore, this type of therapy faces a common issue – high cost. Although the two pharmaceutical companies have not disclosed the price, analysts suggest that, based on the typical pricing of gene therapies, it is likely to be a “million-dollar” level expensive drug.
First CRISPR Gene Editing Therapy Will Revolutionize the Pharmaceutical Industry?
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



