NKG2A Inhibition Reveals a Vulnerability in Triple-Negative Breast Cancer
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Unlocking Immunotherapy’s Potential: NKG2A Inhibition Reveals a Vulnerability in Triple-Negative Breast Cancer
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Unlocking Immunotherapy’s Potential: NKG2A Inhibition Reveals a Vulnerability in Triple-Negative Breast Cancer
Scientists have uncovered a vulnerability in the antigen presentation of triple-negative breast cancer (TNBC), offering a potential breakthrough in immunotherapy resistance.
The research, led by Justin M. Balko and his team at Vanderbilt University in the United States, reveals a weakness in MHC-I heterogeneity in tumors resistant to immunotherapy.
MHC-I, found on the cell surface, is responsible for presenting endogenous antigens to CD8-positive T cells, aiding in the recognition and attack of infected or abnormal cells. Disruption or dysfunction of tumor cell-specific MHC-I (tsMHC-I) is a common mechanism for evading adaptive immunity, leading to resistance to immunotherapy.
However, the study highlights that natural killer (NK) cells, comrades of CD8-positive T cells, do not rely on MHC-I for antigen presentation when attacking tumor cells.
The researchers discovered that the tumor microenvironment with MHC-I heterogeneity recruits a large number of NK cells. Targeting the inhibitory receptor NKG2A expressed by NK cells, in combination with PD-L1 inhibitors, synergistically overcomes resistance to PD-L1 inhibitor therapy. The findings were recently published in the journal “Cancer Discovery.”

The correlation between tsMHC-I levels and the immunotherapy outcomes in breast cancer patients had not been extensively explored before. To investigate, Balko and his team analyzed preoperative biopsy specimens from 84 metastatic TNBC patients participating in a phase II clinical trial (NCT03206203) evaluating the effectiveness of atezolizumab plus carboplatin. Results showed that combination immunotherapy and chemotherapy significantly improved progression-free survival (PFS) for patients with high tsMHC-I levels, while those with low tsMHC-I levels did not benefit additionally from introduced immunotherapy.
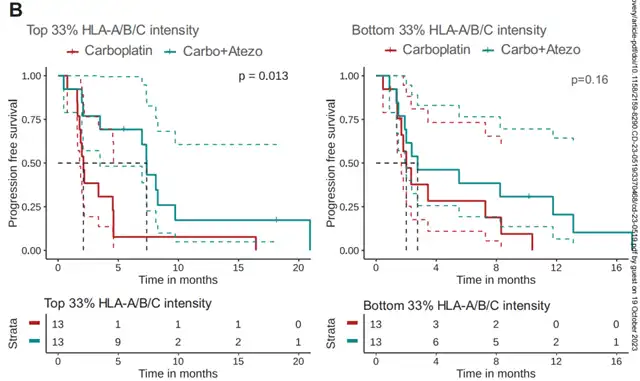
To understand how tsMHC-I affects immunotherapy outcomes, the researchers conducted single-cell analysis of tumor tissue samples from 314 patients with different breast cancer subtypes. They revealed high tsMHC-I heterogeneity in TNBC tumors. Subsequently, mouse experiments showed that the tumor microenvironment with tsMHC-I heterogeneity could recruit higher levels of NK cells in an IFN-γ-dependent manner compared to regions with uniform tsMHC-I expression.
In other words, while tsMHC-I heterogeneity may not be favorable for T cells, it provides an opportunity for NK cells to infiltrate.
To tackle TNBC tumors with tsMHC-I heterogeneity, the researchers proposed mobilizing NK cells. However, NK cells within tumors are not fully effective. Both NK cells and T cells express the inhibitory receptor NKG2A, which produces an immune inhibitory signal when binding to the HLA-E protein expressed by tumor cells. The expression of NKG2A on infiltrating NK cells and T cells in tumors significantly increased compared to normal tissues.
To address this, the researchers used an immune checkpoint inhibitor targeting NKG2A (Monalizumab) for treatment. Results from experiments on breast cancer mouse models (EMT6) showed that single-agent treatment with the NKG2A inhibitor or combined treatment with PD-L1 inhibitor did not improve the survival or inhibit tumor growth in tumor-bearing mice. However, combined therapy demonstrated a significant anti-tumor effect, with a complete response rate of 30%. Similar results were obtained with another breast cancer mouse model (E0771).
Upon testing, on the third day after treatment, mice receiving single-agent NKG2A inhibitor or combined PD-L1 inhibitor treatment showed a significant increase in infiltrating NK cell levels and enhanced cytotoxicity. However, by the seventh day of treatment, only mice receiving combined therapy maintained high levels of infiltrating NK cells in tumors. Additionally, mice receiving PD-L1 inhibitor monotherapy showed increased cytotoxicity in infiltrating T cells but not NK cells.
This indicates that inhibiting NKG2A and PD-L1 has a synergistic effect, activating T cells while awakening a large number of NK cells dormant in the tumor microenvironment with tsMHC-I heterogeneity.
Finally, the researchers evaluated the relationship between tsMHC-I levels and immune cell infiltration in TNBC patients. Results revealed a positive correlation between tsMHC-I levels and CD8-positive T cell infiltration in TNBC tumors, but no linear relationship with NK cell infiltration. Consistent with mouse experiments, tumor regions with tsMHC-I heterogeneity (tsMHC-IHET) had the highest levels of NK cell infiltration and moderate levels of CD8-positive T cell infiltration.
They defined regions with tsMHC-IHET accounting for more than 85% of the patient’s tumor tissue as having high abundance of tsMHC-I heterogeneity. They found that in patients receiving combined chemotherapy and immunotherapy, high abundance of tsMHC-I heterogeneity was closely associated with better progression-free survival, unrelated to the efficacy of chemotherapy alone. Moreover, patients with high abundance of tsMHC-I heterogeneity had a higher ratio of NK cell to T cell infiltration in tumor tissue, suggesting that targeting NKG2A may be more effective for these patients.
In summary, Justin M. Balko and his team found that tsMHC-I heterogeneity alters the pattern of immune cell infiltration. While tsMHC-I heterogeneity hinders T cells from obtaining tumor-specific antigens and exerting cytotoxic functions, it also exposes a fatal weakness by recruiting a large number of NK cells into the tumor microenvironment. This research highlights the potential of NK cells in improving the efficacy of immunotherapy.
Unlocking Immunotherapy’s Potential: NKG2A Inhibition Reveals a Vulnerability in Triple-Negative Breast Cancer
Reference:
[1] https://aacrjournals.org/cancerdiscovery/article/doi/10.1158/2159-8290.CD-23-0519/729449/NKG2A-is-a-Therapeutic-Vulnerability-in
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



