Weight Rebound Challenge After GLP-1 Drug Discontinuation
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New Study Reveals Weight Rebound Challenge After GLP-1 Drug Discontinuation
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
New Study Reveals Weight Rebound Challenge After GLP-1 Drug Discontinuation
New research indicates that more than half of the weight reduced through GLP-1 drugs slowly rebounds after discontinuation.
A study reveals that after a 12-month trial and cessation of Zepbound administration by the company, participants experienced a gradual rebound in weight, with approximately half of the lost weight returning within about eight months.
Eli Lilly and Company (LLY) saw a short-term dip, with an overall decrease of around 2.4% intraday, hitting a daily low of $582.86. Novo Nordisk’s stock price reversed and rose.
GLP-1 drugs have been hailed as a long-awaited panacea for obesity. However, a new study published today exposes the limitations of relying solely on drug intervention to achieve the ideal body mass index.
Glucagon-like peptide-1 (GLP-1) hormones play a crucial role in regulating hunger. Novo Nordisk sells its semaglutide GLP-1 drugs under the labels Ozempic and Wegovy. Eli Lilly sells its tirzepatide GLP-1 under the brand names Mounjaro and Zepbound. These drugs stimulate the pancreas to release insulin, block the unhelpful release of post-meal glucagon, preventing excess glucose from entering the bloodstream, and slow gastric emptying to reduce overall food intake. Additionally, GLP-1 receptors can inhibit the brain’s stress response and reward/reinforcement mechanisms, increasing feelings of fullness. Over time, patients’ daily calorie intake can be reduced by about 20%, leading to significant weight loss.
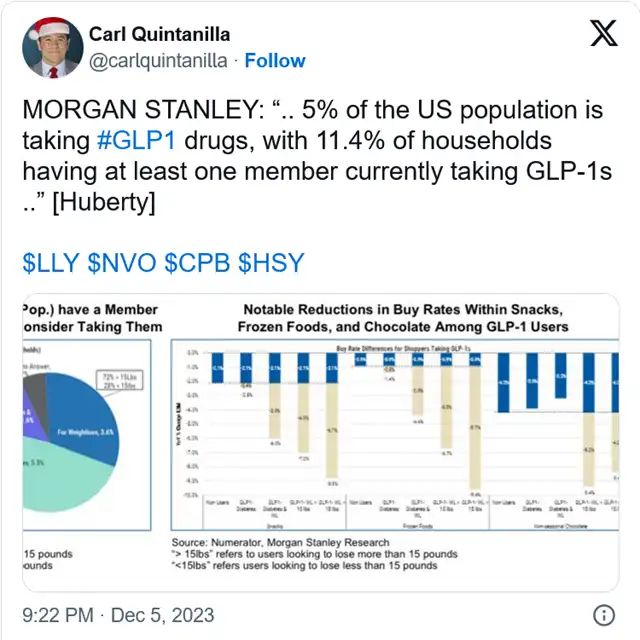
Morgan Stanley recently estimated that about 11% of U.S. households (approximately 5% of the total population) currently have at least one member taking GLP-1 drugs weekly, with another 17% open to the possibility of such intervention for family members.
Capital Group estimates that the total addressable market (TAM) for these GLP-1 drugs far exceeds 2 billion people. This supports predictions that annual sales of these drugs will reach $100 billion by 2030.
A study in the Journal of the American Medical Association states, “From randomization (week 36), patients switched to placebo experienced a 14% weight gain, while those continuing tirzepatide during the 52-week double-blind period had a further weight reduction of 5.5%.”
Here lies the crux of the issue. A recent double-blind study found that after 36 weeks of treatment with Eli Lilly’s tirzepatide GLP-1 product (now sold under the Zepbound label), patients’ average weight decreased by 20.9%. However, after 36 weeks, some patients were randomly switched to a placebo. Those continuing tirzepatide maintained the trajectory of weight loss, while placebo-treated patients regained approximately 14% of their weight, negating most of the weight loss achieved through medication.
These findings suggest that for GLP-1-induced weight loss to be sustained, patients must make a lifelong commitment.
Last week, Eli Lilly announced that its Zepbound GLP-1 drug, within specific coverage limits of commercial health insurance, could be prescribed for as low as $25 per month or three months.
New Study Reveals Weight Rebound Challenge After GLP-1 Drug Discontinuation
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



