Breaking News: Dupixent Shows Potential as an Anti-Cancer Agent
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Breaking News: Dupixent Shows Potential as an Anti-Cancer Agent
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Breaking News: Dupixent Shows Potential as an Anti-Cancer Agent
In a groundbreaking discovery, the original IL-4R antibody Dupixent, developed by Sanofi, initially approved by the FDA in 2017 for treating refractory eczema, has demonstrated promising anti-cancer properties.
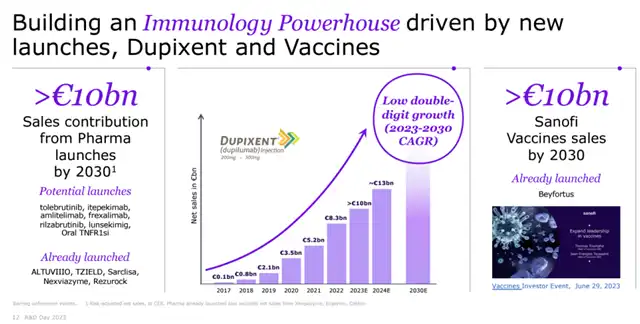
Sanofi and Regeneron plan to submit a chronic obstructive pulmonary disease (COPD) registration application by the end of 2023. Dupixent’s sales for the first three quarters of this year have already reached 7.725 billion euros, showing a 31% year-over-year growth, with expectations to surpass 10 billion euros in sales for the full year 2023.
On December 6th, Nature published a study from the Icahn School of Medicine at Mount Sinai revealing that Dupixent, when combined with PD-1 inhibitors, can slow tumor growth in a mouse model of non-small cell lung cancer (NSCLC). In a small clinical trial involving refractory NSCLC patients, all of whom were administered PD-1 or PD-L1 drugs, one patient experienced near-complete cancer remission.

Dr. Thomas Marron, one of the authors of the study, stated in an interview with Fierce Biotech, “Eighty percent of metastatic lung cancer patients will succumb to this disease, so we must make some improvements… Most importantly, many experimental drugs currently available have strong toxicity and uncertainties, but dupilumab [Dupixent] is already being used by tens of thousands or even millions of people.”
The idea of testing Dupixent in combination with PD-1 in NSCLC originated from early research results published in 2020. At that time, it was believed that cancer hijacked systemic type 2 immune responses, especially IL-4, making them resistant to immune checkpoint inhibitors, preventing effective release of the patient’s T cells to combat cancer.
Marron explained that although there were some early studies on the role of IL-4 in cancer, it was not considered a target. Generally, type 1 immune responses are more correlated with cancer than type 2 immune responses.
“It’s interesting because we traditionally thought IL-4 had nothing to do with cancer,” Marron said. “We thought it was related to asthma, allergies, and all atopic diseases.”
Scientists hypothesized that blocking type 2 immune responses could “rescue” the response to PD-1 drugs. When they validated this theory by blocking IL-4 in mice, they found that not only did it slow tumor growth in mouse models that typically had a mild response to immunotherapy, but it also slowed the growth of tumors in mouse models that usually had no response at all.
Based on these results, Marron concluded that considering Dupixent inhibits both IL-4 and IL-13 and has established safety, it makes sense to explore whether Dupixent has similar benefits for humans. His team conducted an independent clinical trial with six refractory NSCLC patients receiving PD-1. Each received three standard doses at three-week intervals, a longer interval than the current use of Dupixent for asthma or eczema. The primary endpoint of the trial was the response rate at nine weeks.
By the end of the study, biomarker tests for all patients indicated a transition to an immune response to PD-1.
“Suddenly, we saw a strong drive towards type 1 immune response (anti-cancer response),” Marron said. He added that at least half of the patients saw their conditions stabilize, but the remaining patients’ conditions continued to deteriorate. Marron noted that details for some patients were not included in the paper but would be reported at a medical conference next year along with data from a larger cohort.
One case stood out among the participants: a patient who previously had no response to PD-1, but after three doses of Dupixent, the cancer nearly disappeared.
After the study, he continued with immunotherapy, and his cancer continued to shrink. Seventeen months later, his condition remained in remission.
While these early results are promising, there is still much work to be done. Researchers need to determine the optimal dosage and identify the best candidates for the drug.
The ongoing phase 2 clinical trial involves 21 patients and includes blood sample collection and biopsies to help researchers find biomarkers.
“…This may not cure all lung cancers 100%,” Marron said. “I think there may be a subset of patients whose very strong type 2 immune characteristics are the basis for their resistance to immunotherapy, and these patients will benefit from the drug.”
Breaking News: Dupixent Shows Potential as an Anti-Cancer Agent
(source:internetD1Jh6bRVE4bHG3poVEuMsA, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



