Lilly’s drug of weight loss hit new record: Losing 24.2% in 48 weeks
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Lilly’s drug of weight loss hit new record: Losing 24.2% in 48 weeks
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Lilly’s drug of weight loss hit new record: Losing 24.2% in 48 weeks
In the field of drug weight loss, is Lilly itself the one who can beat Eli Lilly?
In June 2022, the FDA approved the marketing of Semaglutide developed by Novo Nordisk as a weight-loss drug.
The drug quickly became popular all over the world that year and achieved sales of more than 10 billion U.S. dollars.
A polypeptide GLP-1 receptor (GLP-1R) agonist. In previous clinical trials, semaglutide had a weight loss effect of 15% in obese patients (injected once a week, each time 2.4mg, for 68 weeks).
In July 2022, Eli Lilly published the results of phase 3 clinical trials of its GIPR/GLP-1R dual agonist Tirzepatide in the New England Journal of Medicine (NEJM) [ 1 ] ,
The results showed that participants lost an average of 22.5% of their body weight after 72 weeks of treatment (15 mg injected once a week) .
This effect not only exceeds the weight loss effect of semaglutide in phase 3 clinical trials, but also breaks the record of weight loss through drugs.
In less than a year, Eli Lilly once again refreshed the drug weight loss record set by itself.
On June 26, 2023, Eli Lilly published a clinical research paper entitled: Triple–Hormone-Receptor Agonist Retatrutide for Obesity — A Phase 2 Trial in the New England Journal of Medicine (NEJM) .
In this phase 2 clinical trial, the GCGR/GIPR/GLP-1R triple agonist Retatrutide achieved a strong weight loss effect over a 48-week treatment period, helping obese subjects lose 24.2% of their body weight at a weekly dose of 12 mg , which is by far the best effect achieved by drug weight loss.

Retatrutide is a triple agonist of the glucose-dependent insulinotropic polypeptide receptor (GIPR) , glucagon-like peptide-1 receptor (GLP-1R) and glucagon receptor (GCGR) developed by Lilly .
In this double-blind, randomized, placebo-controlled phase 2 clinical trial, participants were adults with a BMI ≥ 30, or a BMI between 27 and 30 with at least one weight-related disease (other than diabetes ) .
Participants were randomly assigned to receive subcutaneous injection of Retatrutide in a ratio of 2:1:1:1:2:2, with doses of 1mg, 4mg ( initial dose 2mg) , 4mg (initial dose 4mg), 8mg (initial dose 2mg) , 8mg (initial dose 4mg), 12mg (initial dose 2mg) .
The primary endpoint of the clinical trial was the percent change in body weight from baseline to 24 weeks.
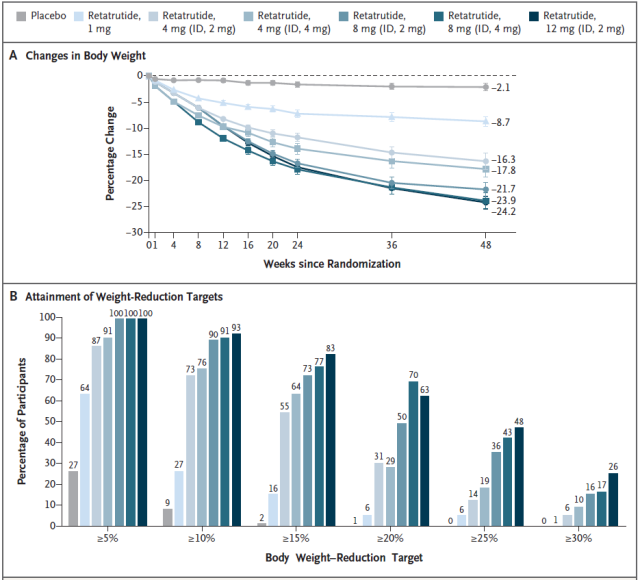
Secondary endpoints included percent weight change from baseline to week 48, proportions with weight loss of ≥5%, ≥10 %, or ≥15%, and safety of treatment.
The clinical trial enrolled 338 adults, 51.8% of whom were men. After 24 weeks of treatment, in the Retatrutide treatment group, the 1mg group lost 7.2%, the 4mg group lost 12.9%, the 8mg group lost 17.3%, the 12mg group lost 17.5%, and the placebo group only 1.6%.
After 48 weeks of treatment, in the Retatrutide treatment group, the 1mg group lost 8.7%, the 4mg group lost 17.1%, the 8mg group lost 22.8%, the 12mg group lost 24.2%, and the placebo group only 2.1%.
After 48 weeks of treatment, the proportions of ≥5%, ≥10 % or ≥15 % in the 4mg dose group were 92%, 75% and 60%, respectively; the proportions of the 8mg dose group were 100%, 91% and 75%, respectively. %; The proportions of the 12mg dose group were 100%, 93% and 83%, respectively . The placebo group was only 27%, 9% and 2%.
The most common adverse events in the retatrutide treatment group were gastrointestinal problems.
These adverse events were dose-related, generally mild to moderate, and adverse events were partially alleviated at lower starting doses.
The dose-dependent increase in heart rate peaked at 24 weeks and gradually declined thereafter.
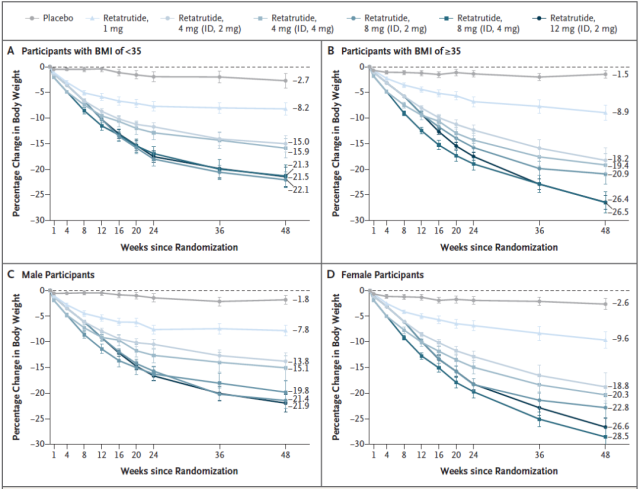
Analysis of the subgroup of treated obese patients showed that Retatrutide treatment had a more significant weight loss effect on obese patients with a BMI ≥ 35, with the 12mg dose reducing body weight by 26.5% at 48 weeks.
In addition, Retatrutide treatment was more effective for weight loss in women , with the 8mg dose leading to a 28.5% weight loss at 48 weeks .
These clinical trial data demonstrate that, in adult obese patients, treatment with Retatrutide resulted in significant weight loss over 48 weeks with a favorable safety profile.
Paper link :
1. https://www.nejm.org/doi/full/10.1056/NEJMoa2206038
2. https://www.nejm.org/doi/full/10.1056/NEJMoa2301972
Lilly’s drug of weight loss hit new record: Losing 24.2% in 48 weeks
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



