Gene Therapy: Advancements and Challenges in 2023
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Gene Therapy: Advancements and Challenges in 2023
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Gene Therapy: Advancements and Challenges in 2023
In 2023, there were groundbreaking advancements in gene therapy in several fields, such as Duchenne muscular dystrophy and hemophilia treatments.
Not only that, but the global number of investigational gene therapy drugs surpassed one hundred for the first time in 2023, reaching 118, the highest ever.
For diseases that traditional drugs cannot treat, gene therapy seems to offer hope of a cure to many patients.
Crossing the darkness, gene therapy drugs are ushering in a dawn of development.
Breakthrough with Over a Hundred New Investigational Drugs
According to public data, there are currently thousands of gene therapy drugs in various stages of development globally, and the number of new additions each year is accelerating.
Table 1 below summarizes the number of new investigational gene therapy drugs added each year over the past decade, based on Pharma Intelligence data.
Table 1: Number of New Gene Therapy Drugs Added Each Year (Incomplete Statistics)
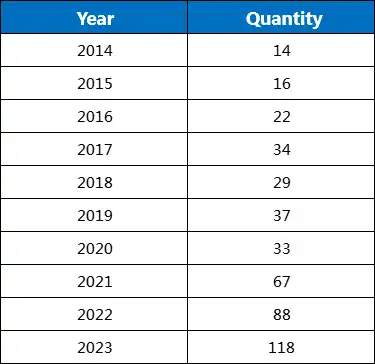
It can be seen that since 2014, the number of investigational gene therapy drugs has been steadily increasing, surpassing one hundred in 2023, reaching 118, the highest ever.
With more gene therapy products being approved for market and rapid advancements in related technologies, gene therapy may be on the brink of a tidal wave of development. The FDA has stated that it expects to approve 10 to 20 gene therapy drugs per year by 2025.
Approved in 2023: Five Major Gene Therapy Drugs
1. Vyjuvek: The First Repeatedly Administered Topical Gene Therapy Drug
Vyjuvek was approved by the FDA in May 2023. Developed by Krystal Biotech, it is used to treat dystrophic epidermolysis bullosa (DEB), a rare and severe genetic skin disease that mainly affects the skin and mucous membranes.
Vyjuvek, which uses the herpes simplex virus as a vector, is the first gene therapy drug in the world that can be repeatedly administered. It is also the FDA’s first drug for treating DEB and the first topical gene therapy drug. Most gene therapy drugs currently use adenovirus-related vectors, while Vyjuvek uses the herpes simplex virus as a vector, marking a significant step forward in gene therapy.
Clinical data shows that after 24 weeks of treatment, 65% of wounds treated with Vyjuvek were completely healed, compared to 26% in the placebo group.
In terms of the market, according to Krystal Biotech’s third-quarter 2023 report, as of September 30, 2023, the company had received 284 applications for medication. The company plans to report the number of patients treated starting in the first quarter of 2024 to better predict Vyjuvek’s sales.
2. Elevidys: The World’s First Duchenne Muscular Dystrophy Gene Therapy Drug
Developed by Sarepta, Elevidys was approved by the FDA in 2023. It is the world’s first gene therapy drug for treating Duchenne muscular dystrophy, targeting children aged 4-5 years old.
Phase 1/2 clinical data shows that after 52 weeks of use, patients’ NSAA scores improved by 3.8 points. Four years later, the average difference in NSAA scores among patients reached 7.0 points.
In terms of pricing, Elevidys is priced at $3.2 million per dose. Despite its high price, Elevidys performed well in sales in 2023, with total sales for the year reaching approximately $200 million, or about 1.4 billion yuan.
3. Roctavian: The World’s First Hemophilia A Gene Therapy Drug
Developed by BioMarin, Roctavian was approved on June 29, 2023. It is the world’s first gene therapy drug for treating hemophilia A.
Clinical data shows that after receiving Roctavian treatment, patients’ annualized bleeding rates can be reduced by 52%. Compared to traditional prophylactic FVIII treatment, patients treated with Roctavian experience fewer bleeding episodes. The spontaneous and joint bleeding rates for traditional FVIII prophylactic treatment are 2.3 and 3.1 times per year, respectively, while for Roctavian treatment, they are 2.3 and 3.1 times per year, respectively. Additionally, according to the phase 3 GENEr8-1 results, patient bleeding volumes decreased by 82.9%.
In terms of pricing, the drug is priced at $2.9 million in the United States. In the market, according to BioMarin’s third-quarter 2023 financial report, Roctavian sales were $822,000, which is currently lagging behind Elevidys’ sales. However, as the drug has only been on the market for a short time, its future performance remains uncertain. It is predicted that the drug’s peak sales could reach $2.2 billion.
4. Casgevy: The First Gene Therapy Drug Based on CRISPR/Cas9 Gene Editing Technology
Casgevy is a CRISPR/Cas9 gene editing therapy developed jointly by Vertex and CRISPR. It was approved in the UK in November 2023 and in the US in December 2023. It is the world’s first gene therapy drug based on CRISPR/Cas9 gene editing technology and is used to treat recurrent vascular occlusion in sickle cell disease patients aged 12 and above.
Clinical data shows that among patients treated with Casgevy, 29 out of 31 patients did not experience serious vascular occlusion events for at least 12 consecutive months, with an effectiveness rate of 93.5%.
5. Lyfgenia: The FDA’s Approved Twin
Two gene therapy drugs with the same indication were approved by the FDA on the same day: Casgevy and Lyfgenia. Lyfgenia, developed by bluebird bio, is also used for sickle cell disease patients aged 12 and above with recurrent vascular occlusion.
Clinical data shows that after using Lyfgenia, 94% of patients did not experience serious vascular occlusion, and 88.2% did not experience vascular occlusion events, showing remarkable effectiveness. However, in terms of adverse reactions, the use of Lyfgenia may lead to malignant blood tumors in patients, resulting in an FDA black box warning.
Lyfgenia is priced higher than Casgevy, at $3.1 million. As both drugs have the same indication and were approved on the same day, the FDA’s move has also attracted attention. It remains to be seen who will stand out in the future.
Bright Future but Two Major “Roadblocks” to Overcome
High Prices
It is well known that gene therapy drugs often come with sky-high prices, which are not only difficult for patients to accept but also beyond the capability of national health insurance to bear. However, the high pricing of gene therapy drugs is not the intention of the companies; rather, it is due to the excessively high research and development costs of gene therapy drugs.
The core of gene therapy drugs is the viral vector, and its high production cost is also one of the main reasons for the high pricing of gene therapy drugs. It is also an important factor restricting the industry’s future development.
Safety Concerns
Although gene therapy has shown strong potential in treating genetic diseases, safety is also a major concern. Because gene therapy drugs often involve novel technologies compared to traditional drugs, there is a great deal of uncertainty due to the lack of a mature non-clinical research evaluation system. One of the future directions for development is to establish a mature drug development system to ensure the safety of drugs to a greater extent.
Compared to traditional drugs, gene therapy drugs have their unique “charm,” but high prices and safety concerns are “roadblocks” that hinder patients from using them. It is believed that after these two major issues are resolved, the gene therapy drug market will experience a major breakthrough.
Gene Therapy: Advancements and Challenges in 2023
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



