The Key Patents of 10 Blockbuster Drugs to Expire in the Next 5 Years
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
The Key Patents of 10 Blockbuster Drugs to Expire in the Next 5 Years
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
The Key Patents of 10 Blockbuster Drugs to Expire in the Next 5 Years
Patent protection is a key weapon for the sustained development of a drug or even an entire company, a fact most evident in AbbVie’s Humira.
Over the next five years, key patents for several blockbuster drugs are set to expire, presenting significant business opportunities for the pharmaceutical industry, especially for generic and biosimilar developers.
Here are the 10 blockbuster drugs with key patents expiring in the next 5 years, which collectively generated over $80 billion in total sales in 2023.
Table 1: 10 Blockbuster Drugs with Key Patents Expiring in the Next 5 Years
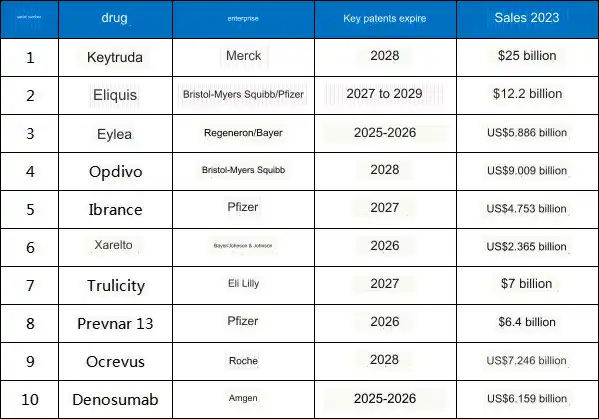
Note: The sales figure for the eighth drug includes the combined sales of Prevnar 13 and Prevnar 20.
01. Keytruda
- Active Ingredient: Pembrolizumab
- Developer: Merck
- Indications: Triple-negative breast cancer, melanoma, non-small cell lung cancer, head and neck squamous cell carcinoma, urothelial carcinoma, colon cancer, or rectal cancer.
- 2023 Sales: $25 billion
- Key Patent Expiry: 2028
Keytruda was first approved by the FDA in 2014 for the treatment of various types of cancer. Since its initial approval, Keytruda’s approved indications have continued to expand, including melanoma, non-small cell lung cancer, head and neck cancer, among others.
In 2023, Keytruda accounted for 40% of Merck’s drug sales, making it the company’s most successful product. Keytruda is expected to lose patent protection in 2028, opening the door to competition from biosimilars.
Sales of Keytruda are projected to decrease to $27.4 billion by 2029, a 19% decrease from the projected $33.7 billion in sales for 2028. To offset the significant revenue decline, Merck is preparing to strengthen its immunotherapy product portfolio by establishing an early asset pipeline. Additionally, Merck reached a final agreement in early 2024 to acquire Harpoon Therapeutics for $680 million, further advancing its oncology product portfolio.
02. Eliquis
- Active Ingredient: Apixaban
- Developers: Bristol-Myers Squibb and Pfizer
- Indications: Reducing the risk of stroke or blood clots in patients with atrial fibrillation
- Key Patent Expiry: 2027 to 2029
- 2023 Sales: $12.2 billion
Eliquis is a drug used to prevent blood clots in adults. Eliquis was first approved in May 2011 in the EU and in December 2012 in the US and has become one of the most effective treatment options for deep vein thrombosis and pulmonary embolism. Eliquis was the top-selling drug in the cardiovascular disease sector in 2023 ($12.2 billion), far surpassing Novartis’s Entresto ($5.8 billion) in second place. Eliquis is expected to maintain this position until 2028.
With Eliquis’s patents set to expire in the coming years, generic versions of Eliquis have already received temporary approval from the FDA. Due to a settlement and a key lawsuit, these two pharmaceutical giants are able to hold onto Eliquis’s market position at least until 2027.
It is currently unclear when exactly Eliquis generics will enter the market, but it is unlikely to be before 2027. The FDA has granted an extension for Eliquis’s pediatric use, which will extend its market exclusivity by six months.
03. Eylea
- Active Ingredient: Aflibercept
- Developers: Regeneron, Bayer
- Indications: Wet age-related macular degeneration and other eye diseases
- Key Patent Expiry: 2025 to 2026
- 2023 Sales: $5.886 billion (US)
Eylea was approved by the FDA in 2011 for the treatment of wet age-related macular degeneration, diabetic retinopathy, and diabetic and non-diabetic macular edema. In 2023, Eylea’s sales in the US reached $5.89 billion. Experts predict that Eylea’s patent will expire in 2025 or 2026, although Regeneron has claimed to investors that their market exclusivity may be longer. Meanwhile, major pharmaceutical companies such as Sandoz and Viatris have targeted market share, actively developing biosimilars to capture the market.
04. Opdivo
- Active Ingredient: Nivolumab
- Developer: Bristol-Myers Squibb
- Indications: Various cancers
- Key Patent Expiry: 2028
- 2023 Sales: $9.009 billion
Bristol-Myers Squibb’s Opdivo (nivolumab) is an immunotherapy used to treat various cancers and is a direct competitor to the blockbuster drug Keytruda. While its sales are less than half of Keytruda’s, it still achieved sales of $9.009 billion in 2023, making it a significant blockbuster drug.
Thanks to approvals for some new indications, Opdivo has shown impressive growth in recent years, with sales growing 9% in 2023, and sales are expected to reach $11.75 billion by 2026. However, BMS will lose its exclusive market rights for Opdivo in the US in 2028.
Sydney-based NeuClone Pharmaceuticals, Swedish company Xbrane Biopharma, and China’s Green Valley Pharmaceuticals are already working on developing biosimilars of nivolumab. Several blockbuster drugs are set to lose their patents involving BMS in the coming years, including not only Opdivo but also the cancer drug Revlimid and Eliquis, a blood thinner developed in collaboration with Pfizer. BMS is currently working to launch six new drugs, which together could contribute over $15 billion in sales.
05. Ibrance
- Active Ingredient: Palbociclib
- Developer: Pfizer
- Indications: HR-positive, HER2-negative breast cancer
- Key Patent Expiry: 2027
- 2023 Sales: $4.753 billion
While Pfizer expects their anti-cancer drug Ibrance to maintain its US market exclusivity until 2027, this has not stopped generic drug companies from developing their own Palbociclib generics.
Several generic drug manufacturers notified Pfizer in 2019 that they had submitted applications for generic versions of Ibrance to the FDA. Although they argued that two key patents for Ibrance were invalid, Pfizer was successful in obtaining a 4-year extension until 2027. In fact, sales of Ibrance already declined in 2023, dropping from $5.12 billion a year earlier to $4.753 billion, mainly due to competitive pressure and reduced clinical trial procurement in some international markets leading to decreased global demand. With uncertainty surrounding the future of Ibrance, Pfizer has been working to strengthen its oncology product portfolio over the past few years, including deals to acquire Array BioPharma, Seagen, and Trillium Therapeutics.
06. Xarelto
- Active Ingredient: Rivaroxaban
- Developers: Bayer/Janssen
- Indications: Blood clots
- Key Patent Expiry: 2026
- 2023 Sales: $2.365 billion
Xarelto (Rivaroxaban) was patented in 2007 and approved for medical use in the US in 2011, for the treatment and prevention of blood clots. This anticoagulant, developed by Bayer and Janssen Pharmaceuticals under Johnson & Johnson, has been prescribed over 80 million times in the US alone. In 2022, it was Bayer’s best-selling drug, generating revenues of €4.516 billion.
Bayer is expected to lose exclusivity for Xarelto in 2026. In the US market, Lupin’s generic version of Rivaroxaban (RLD Xarelto) has already received tentative FDA approval and is actively preparing to launch its generic. With both Xarelto and Eylea, two of its star products, set to expire, Bayer has been searching for new growth opportunities and focusing on further expanding its development portfolio in the areas of cell and gene therapy.
07. Trulicity
- Active Ingredient: Dulaglutide
- Developer: Lilly
- Indications: Type 2 diabetes
- Key Patent Expiry: 2027
- 2023 Sales: $7 billion
Trulicity is an injectable used to treat type 2 diabetes in adults and children aged 10 and older.
Trulicity was approved in 2014, and its active ingredient dulaglutide’s patent protection is set to expire in 2027, opening the way for generic drug makers.
Trulicity became the 17th top-selling drug globally in 2023. With the introduction of tirzepatide formulations Mounjaro and Zepbound, the gradual decline of Trulicity is expected to be smoothly resolved for Lilly.
08. Prevnar 13
- Developer: Pfizer
- Indications: Infections caused by Streptococcus pneumoniae strains
- Key Patent Expiry: 2026
- 2023 Sales: $6.4 billion (combined sales of Prevnar 13/20)
Pfizer’s Prevnar 13 is a vaccine used to prevent infections caused by 13 strains of Streptococcus pneumoniae bacteria, which can lead to pneumonia, meningitis, and other serious diseases. Prevnar 13 was first used in infants in Europe at the end of 2009 and was then approved by the FDA in February 2010. In 2015, the US federal government recommended the vaccine for people over 65, bringing Pfizer huge profits.
Prevnar 13’s patent is expected to expire in 2026. To offset the loss from the expiration of the Prevnar 13 patent, Pfizer has invested heavily in research and development, receiving 7 FDA approvals in 2023 and acquiring Seagen Inc in December 2023 to strengthen its oncology product portfolio.
09. Ocrevus
- Active Ingredient: Ocrelizumab
- Developer: Roche
- Indications: Relapsing or primary progressive multiple sclerosis
- Key Patent Expiry: 2028
- 2023 Sales: CHF 6.381 billion ($7.246 billion)
Roche’s multiple sclerosis drug Ocrevus (Ocrelizumab) for relapsing or primary progressive multiple sclerosis was their second top-selling asset in 2023, with sales of CHF 6.381 billion ($7.246 billion). Ocrevus’s patent in the US is expected to expire in 2029, with patents in Germany, France, Spain, the UK, and Italy expiring in 2028. Many life science companies are racing to develop biosimilars of Ocrelizumab, including South Korean biopharmaceutical company Celltrion, which announced 3rd phase approval in 2023.
Roche remains focused on internal development, recently announcing promising results for their new breast cancer treatment inavolisib. They are also increasing their investments in projects and corporate acquisitions to strengthen their portfolio in areas such as inflammatory bowel disease and cardiovascular metabolic diseases.
10. Prolia/Xgevav
- Active Ingredient: Denosumab
- Developer: Amgen
- Indications: Secondary bone cancer
- Key Patent Expiry: 2025 to 2026
- 2023 Sales: $4.048 billion (Prolia), $2.111 billion (Xgeva)
Prolia/Xgeva (Denosumab) was approved by the FDA in 2010 for the treatment of secondary bone cancer. In some regions of Europe, Prolia/Xgeva has already lost patent protection, and its patents in major markets such as the US, France, Italy, Spain, and the UK are set to expire between 2025 and 2026.”
The Key Patents of 10 Blockbuster Drugs to Expire in the Next 5 Years
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



