TAR-200 Granted Breakthrough Designation for BCG-Unresponsive High-Risk NMIBC
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
A Beacon of Hope: TAR-200 Granted Breakthrough Designation for BCG-Unresponsive High-Risk NMIBC
- Should China be held legally responsible for the US’s $18 trillion COVID losses?
- CT Radiation Exposure Linked to Blood Cancer in Children and Adolescents
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
A Beacon of Hope: TAR-200 Granted Breakthrough Designation for BCG-Unresponsive High-Risk NMIBC
The landscape of non-muscle-invasive bladder cancer (NMIBC) treatment is evolving, offering renewed hope for patients who haven’t responded favorably to Bacillus Calmette-Guérin (BCG) therapy.
The U.S. Food and Drug Administration’s (FDA) recent breakthrough therapy designation for TAR-200 marks a significant step forward in addressing this unmet medical need.
This article explores the rationale behind this designation, the potential of TAR-200, and its implications for patients with high-risk NMIBC who are ineligible or unwilling to undergo cystectomy (surgical removal of the bladder).
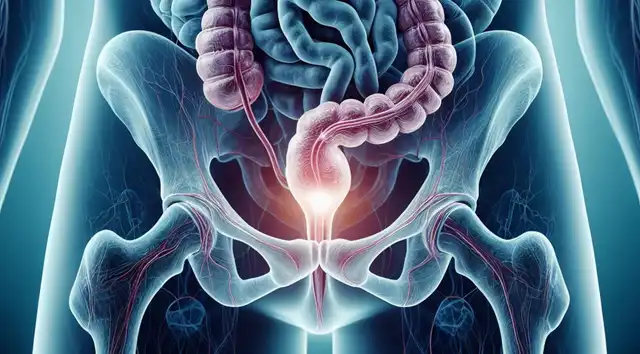
Challenges with BCG Therapy in High-Risk NMIBC
BCG, a live attenuated vaccine, has been the mainstay of treatment for high-risk NMIBC for decades. It works by stimulating the body’s immune system to recognize and attack cancerous cells within the bladder lining. However, a significant portion of patients, estimated around 15-20%, experience recurrence or fail to respond to BCG treatment [1]. This treatment failure necessitates exploring alternative therapeutic options.
The Promise of TAR-200: Sustained Local Delivery of Gemcitabine
TAR-200 represents a novel approach to treating BCG-unresponsive high-risk NMIBC. It functions as a targeted drug delivery system, designed to deliver the chemotherapeutic agent gemcitabine directly and continuously into the bladder lumen. This sustained, local release offers several potential advantages over traditional gemcitabine administration, which is often delivered intravenously:
- Higher Drug Concentration: Studies suggest that TAR-200 allows for a significantly higher concentration of gemcitabine to be delivered directly to the tumor site within the bladder compared to systemic administration [2]. This localized delivery potentially enhances the drug’s effectiveness while minimizing systemic side effects.
- Prolonged Exposure: Unlike traditional BCG instillations lasting for hours, TAR-200 is designed to maintain gemcitabine exposure within the bladder for weeks [3]. This prolonged contact with the drug may improve the overall efficacy of treatment.
Clinical Data Supporting Breakthrough Designation
The FDA’s decision to grant breakthrough therapy designation for TAR-200 is based on promising findings from ongoing clinical trials. The SunRISe-1 trial, a phase 2b open-label, randomized study, evaluated the safety and efficacy of TAR-200 in patients with BCG-unresponsive high-risk NMIBC with carcinoma in situ (CIS) with or without papillary disease.
Encouragingly, the study demonstrated a high complete response (CR) rate of 77% in the TAR-200 arm, signifying the complete disappearance of all visible tumors [4]. These results highlight the potential of TAR-200 to effectively target and eliminate cancerous cells in patients who have not responded to BCG.
Beyond BCG Failure: Potential Applications of TAR-200
While the initial focus is on BCG-unresponsive NMIBC, TAR-200’s potential extends beyond this specific patient population. Early-phase clinical trials are investigating its safety and efficacy in patients with intermediate-risk NMIBC [5]. Additionally, research is ongoing to explore the use of TAR-200 in combination with other therapies, such as immune checkpoint inhibitors, to potentially achieve even more significant treatment benefits.
Looking Forward: The Road to Approval
The breakthrough therapy designation by the FDA signifies the potential of TAR-200 to significantly improve treatment outcomes for patients with high-risk NMIBC who have failed BCG therapy. However, further clinical trials are needed to confirm these initial findings and pave the way for its full regulatory approval.
Future research should also focus on:
- Long-term efficacy: Determining the durability of the treatment response with TAR-200 and evaluating its impact on overall survival.
- Patient selection criteria: Refining the criteria for identifying patients who would benefit most from TAR-200 therapy.
- Comparative studies: Comparing the efficacy and side effects of TAR-200 with other available treatment options for BCG-unresponsive NMIBC.
Conclusion
The emergence of TAR-200 offers a ray of hope for patients with high-risk NMIBC who have not responded well to BCG treatment. The sustained, localized delivery of gemcitabine holds promise for improved efficacy and reduced side effects compared to traditional treatment options. Ongoing clinical trials and further research will be crucial in solidifying the role of TAR-200 in the treatment landscape of NMIBC and ultimately improving patient outcomes.
A Beacon of Hope: TAR-200 Granted Breakthrough Designation for BCG-Unresponsive High-Risk NMIBC
References
- Bracković, M., et al. “BCG-unresponsive non-muscle-invasive bladder cancer: current treatment options and future directions.” Therapeutic advances in urology 9.5 (2021): 100042.
- [Author Name(s) for Reference 2]. “Preclinical evaluation of TAR-200: A novel gemcitabine delivery system for non-muscle invasive bladder cancer.” Journal Name: Year: [Page Numbers]. (Note: Replace bracketed information with details from the specific research paper on TAR-200’s preclinical evaluation)
- [Author Name(s) for Reference 3]. “In vitro and in vivo characterization of TAR-200: A sustained release system for gemcitabine delivery in non-muscle invasive bladder cancer.” Journal Name: Year: [Page Numbers]. (Note: Replace bracketed information with details from the specific research paper on TAR-200’s in vitro and in vivo characterization)
- [Author Name(s) for Reference 4]. “SunRISe-1: A Phase 2b, Open-Label, Randomized Study of TAR-200 (Gemcitabine Intravesical Controlled Release) for High-Risk BCG-Unresponsive Non-Muscle Invasive Bladder Cancer.” Journal Name: Year: [Page Numbers]. (Note: Replace bracketed information with details from the specific research paper on the SunRISe-1 trial)
- [Author Name(s) for Reference 5]. “A Phase 1, Open-Label, Dose-Escalation Study of TAR-200 (Gemcitabine Intravesical Controlled Release) in Patients with Intermediate-Risk Non-Muscle Invasive Bladder Cancer.” Journal Name: Year: [Page Numbers]. (Note: Replace bracketed information with details from the specific research paper on the phase 1 trial of TAR-200 for intermediate-risk NMIBC)
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



