The world’s first Medtronic’s Harmony TPV valve Approved by FDA!
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
The world’s first Medtronic’s Harmony TPV valve Approved by FDA!
The world’s first Medtronic’s Harmony TPV valve approved by FDA!. On March 26, the US Food and Drug Administration (FDA) approved the Medtronic Harmony™ Transcatheter Pulmonary Valve (TPV).

According to official FDA news, Medtronic’s Harmony™ Transcatheter Pulmonary Valve (TPV) is the world’s first non-surgical heart valve. This product is used to treat congenital heart disease and is specifically designed for severe pulmonary valve insufficiency (blood leaking back into the lower right chamber of the heart).
“Chestectomy” with high mortality
Congenital heart disease (CHD) is the most common congenital disease. Approximately 40,000 babies suffer from the disease each year, an increase of 18.7% from 1990 to 2017. According to public data, in the United States, more than 2 million babies, children, adolescents, and adults suffer from congenital heart disease. Patients with congenital heart disease usually need heart surgery shortly after birth, and throughout their lives It needs to be done multiple times.
According to Matthew J. Gillespie, the main clinical researcher of Medtronic’s Harmony™ TPV transcatheter pulmonary valve, patients with congenital heart disease will face a large number of open heart operations throughout their lives to continuously treat their pulmonary valve problems. In addition, patients with congenital heart disease need life-long cardiac preventive monitoring, health care, and special treatment.
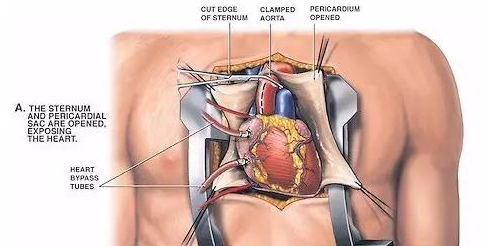
However, patients after heart surgery are likely to have symptoms such as severe pulmonary valve insufficiency, leading to pulmonary blood reflux. In the past, severe pulmonary valve insufficiency was treated by open heart surgery and other operations, and the abnormality was corrected in the bloodless heart through the heart pump and artificial lung to restore self-circulation, but because of the risk of cardiac ischemia and blood reperfusion injury And so on, the operation has a higher mortality rate.
Medtronic “Minimally Invasive Alternative”
Harmony™ TPV transcatheter pulmonary valve provides a minimally traumatic alternative to surgical thoracotomy for patients with congenital heart disease during surgery.
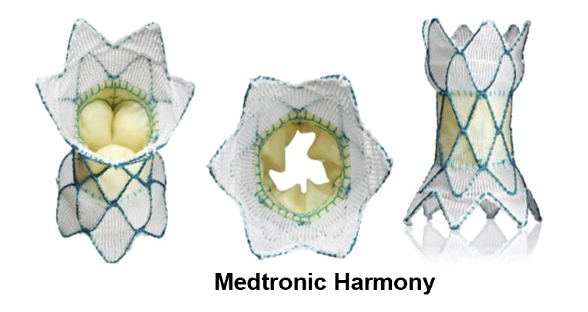
During Medtronic Harmony™ transcatheter pulmonary valve implantation, a thin hollow tube (catheter) with the product folded at the end is inserted into the right side of the heart through a vein in the groin or neck, and then the valve is released from the catheter. The product can expand by itself and anchor it to the right ventricular outflow tract. After the heart is installed in place, it will open and close like a door, allowing blood to flow in the right direction.
Principle of valve product implantation (source Medtronic)
Years of clinical research
It is worth noting that this product can improve the blood flow to the lungs of patients with severe pulmonary valve insufficiency in the case of non-open heart surgery, greatly improving the safety of the operation.
At the 65th Annual Scientific Meeting of the American College of Cardiology (ACC) in 2016, Medtronic announced for the first time the results of the early feasibility study of the Harmony transcatheter pulmonary valve (TPV). According to the 6-month follow-up results, 18 cases of Harmony implantation The blood reflux rate of patients with severe pulmonary valve insufficiency of TPV dropped from 95% to 0.
Currently, the FDA evaluates the safety and effectiveness of the Harmony TPV valve through research. It is understood that in the course of this study, doctors implanted the device in 70 patients, and performed follow-up inspections when the patients were discharged from the hospital and the first month, six months, and from every year to five years after implantation. The main safety index is that there will be no deaths related to surgery or equipment within 30 days after implantation, and the results show that all patients have achieved it.
The data from the study also showed that all patients treated with the Harmony TPV valve achieved the main effectiveness index that no additional surgical procedures or interventional procedures related to the device were required at 6 months.
According to Bram Zuckerman, director of the FDA’s Center for Radiological Health and Office of Cardiovascular Devices, Medtronic’s Harmony™ TPV transcatheter pulmonary valve provides a new treatment option for congenital heart disease, which is for leaking or surgically repaired right ventricular outflow. Dao (heart malformation) patients provide a non-invasive alternative to open surgery, which can help patients improve people’s quality of life and resume their normal activities more quickly, thereby meeting the clinical needs of many patients with congenital heart disease .
At present, the Harmony TPV valve has passed the (PMA) pre-sale license, and will be put into market use next. In order to further ensure the safety and effectiveness of the Harmony TPV valve, the study has extended the follow-up investigation time of study patients to 10 years after the product approval. In the future, it will continue to iterate products to provide a wider range of choices for the majority of congenital heart disease patients.
(source:internet, reference only)
Disclaimer of medicaltrend.org



