New technology for transcatheter pulmonary valve replacement
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
New technology for transcatheter pulmonary valve replacement
New technology for transcatheter pulmonary valve replacement. Complex congenital heart disease (such as tetralogy of Fallot, pulmonary atresia, etc.) with low pulmonary blood type often has residual pulmonary regurgitation (PR) after the first corrective surgery.
This is the case after this type of surgery (especially if a trans-ring Patch repair technique) the most common long-term complication. Severe PR can cause right ventricular outflow tract (RVOT) tumor-like dilation and dysfunction, pulmonary aneurysm-like dilation, ventricular asynchrony and arrhythmia, progressive right ventricular dilatation/right ventricular dysfunction, and subsequent right heart failure, eventually Causes heart failure and death. In recent years, the significance of pulmonary valve reconstruction has received more and more attention.
Traditional surgical pulmonary valve replacement requires reopening of the chest, which is performed under cardiopulmonary bypass. Artificial valve types (including biological valve, mechanical valve and valved pipeline) have many applications. Transcatheter pulmonary valve replacement (TPVR) is a minimally invasive surgery method that has gradually emerged since Bonhoeffer’s first report in 2000. It can replace surgical pulmonary valve replacement and improve the long-term prognosis of such patients. Although transcatheter pulmonary valve replacement was first available, it was affected by the number of patients, indications, durability and promotion value, and its development lags behind the transcatheter treatment of left heart valve disease. This article describes the development status and future prospects of TPVR.
T
1. Selection of surgical procedures for tetralogy of Fallot and complex anatomy of the long-term right ventricular outflow tract
The first corrective surgery for tetralogy of Fallot in infants or childhood is usually to dredge or reconstruct RVOT through various methods to relieve pulmonary artery stenosis, but it can lead to immediate or delayed progressive PR. According to the extent and scope of the disease, the specific surgical procedures for tetralogy of Fallot can be divided into RVOT incision and dredging, RVOT patch, pulmonary valve and annulus incision, valve resection, different range of transvalvular patch, and valve span Ring patch (homemade), replacement of the same or heterogeneous valved pipeline, PTFE artificial double valve pipeline, right ventricle-external pulmonary artery pipeline, RVOT dual channel, etc. The postoperative RVOT anatomy is diverse, plus long-term PR caused Chronic RVOT and pulmonary aneurysm-like expansion make its anatomy more complicated.
Infundibular preservation via the right atrium/transpulmonary artery can improve mid-term atrioventricular function and reduce the risk of arrhythmia, but there is still a lack of clear long-term longitudinal comparison results for different surgical approaches. Pulmonary valve preservation technology can theoretically maintain long-term valve function, but it will cause stenosis and persistent right ventricular hypertension.
Complete transright ventricular repair combined with pulmonary valvuloplasty can optimize the growth of the annulus, but its long-term benefits have not been proven. The RVOT incision technique is also often affected by the variation of the coronary artery that spans the outflow tract. The single leaflet trans-annular patch repair technique often results in late pulmonary valve function loss. At present, there is no consensus regarding the radical surgery for patients with tetralogy of Fallot, the best surgical method and timing, but when the surgical method is formulated, attention should be paid to the preservation of right ventricular function as much as possible, and the long-term pulmonary valve and right ventricular outflow tract anatomy should be fully considered And function, and reduce the possibility of arrhythmia.
After tetralogy of Fallot, patients with RVOT have various anatomical characteristics. Schievano et al. classified them morphologically and defined five schematic RVOT geometric shapes, including positive “eight”, reverse “eight”, and straight. Type, shuttle type, dumbbell type. Although the morphological types are simplified, there is still extensive variability in each type. In addition, no direct correlation was found between the surgical history or underlying pathology and the subsequent RVOT morphological classification, confirming its unpredictability and wide variability.
In view of the anatomical structure of the implantation site, a good riveting of the stent must be provided to ensure the stability and function of the transcatheter valve. Different types and diameters of RVOT have an important influence on the judgment of the indications for implantation, the determination of the implantation site and method, and the choice of valve model. Except that the autologous RVOT is often too large in size, the size of the RVOT has great variability throughout the cardiac cycle.
The RVOT cross-sectional area and diameter (including the major and minor diameters) can vary by more than 50%, and the axial length variability ( Shorten and extend) can be as high as 80%. Some patients have local tumor-like structures, asymmetrical expansion, irregular geometric shapes, and good compliance with large differences, which also increases the complexity and unpredictability of the dynamic structure of RVOT.
The secondary surgical treatment of the previous surgical pulmonary valve includes mechanical or biological pulmonary valve replacement, enlarged PTFE tubing with artificial bi-leaflet valve, homologous valved tubing (aortic or lung allograft), xenogeneic valved tubing (Stentless pig flap, artificial tube sutured with pig flap, bovine jugular vein) replacement, etc. Bioprosthetic valves and valved catheters will eventually develop progressive valve dysfunction within 10 to 20 years after implantation.
At the same time, the risk of infective endocarditis must be weighed. The durability of the traditional biological porcine valve is similar to that of the pericardial valve, and it is often used as the first choice in adult surgery, and is beneficial to the future transcatheter pulmonary valve replacement. Theoretically speaking, the same kind of valved tubing has the best durability and is suitable for infant surgery, and its durability in the pulmonary position is better than that in the extracardiac position (right ventricle to pulmonary artery incision).
The durability of the bovine jugular vein in infants and young children is similar to that of allografts, so it is also widely used. However, the durability of the same kind of valved catheter in adulthood is lower than that of standard bioprostheses and non-stent bioprostheses. Some new types of catheters lack long-term data, especially extracardiac catheters, which should be avoided as much as possible because they are difficult to perform percutaneous valve replacement. Although mechanical pulmonary valve replacement has a low rate of valve deterioration, it requires life-long systemic anticoagulation therapy to avoid thrombosis.
However, due to the various limitations or contraindications of systemic anticoagulation in young people and adolescents, and the access limitation of pulmonary artery branch interventional therapy, etc. There are few practical applications, unless combined with left heart mechanical valve replacement or other diseases that require long-term anticoagulation.
2. The characteristics and limitations of balloon expandable valves
At present, the internationally mature balloon-expandable transcatheter pulmonary valves include the Melody valve system (United States) and the Sapien valve system (United States). There have been 10,000 implants worldwide (most of which use Melody valves). Due to the widespread use of valved pipeline surgical strategies in Europe and America, the long-term restenosis (± regurgitation) is the mainstay.
The design concept of balloon dilatation valve products is to reconstruct the insufficient pulmonary valve in the right ventricular outflow tract-pulmonary valved pipeline , Has become the standard method for the treatment of postoperative pulmonary valve stenosis with regurgitation. Due to the small valve model, it is not suitable for the anatomical characteristics of RVOT, which has tumor-like expansion due to long-term PR, and neither of the above two products has been clinically applied or marketed in China.
2. 1 Melody valve Melody valve is the first commercially available valve to be marketed.
It obtained CE Mark certification in 2006, entered the United States in 2010, and obtained pre-market approval from the U.S. Food and Drug Administration (FDA) in 2015. In 2017 It is officially approved for transcatheter pulmonary valve therapy and valve-in-valve placement. Melody valve is developed from the initial device used by Bonhoeffer et al. It is to suture the bovine jugular vein valved tube (tri-leaflet valve) along the stent to the platinum-iridium alloy stent carrier, and each node on the braided metal wire of the stent Welded into a closed unit by gold filler (Figure 1).
The stent is 28 mm long and 6 mm in diameter after compression. The outer diameter of the valve is approximately 2 mm than the inner diameter, and the inner diameter of the valve can be expanded to 18 mm, 20 mm, or 22 mm according to the balloon size (corresponding to the stent length after expansion 23 mm, 24 mm, 26 mm). The initial intervention criteria included RVOT-pulmonary artery duct diameter> 16 mm, balloon measurement narrowest diameter of 14-20 mm, moderate to severe PR or stenosis (pressure difference> 35 mmHg, 1 mmHg=0.133 kPa).
It has been reported that a 24 mm balloon can be used to expand the stent as indicated above. If it is expanded under high pressure (> 4 atm, 1 atm = 101.325 kPa), it can be expanded to an inner diameter of 25 mm. At present, the Melody valve has been widely used in the treatment of right ventricular-pulmonary duct dysfunction and biological valve failure. It is also used in a part of the autologous outflow tract PR with a small annulus diameter. The autologous outflow tract implantation requires improved implantation including pre-stents. Technology, because valve placement usually requires a certain circular riveting area, and for TPVR in the right ventricle-pulmonary artery (such as Rastelli surgery), there are also reports.
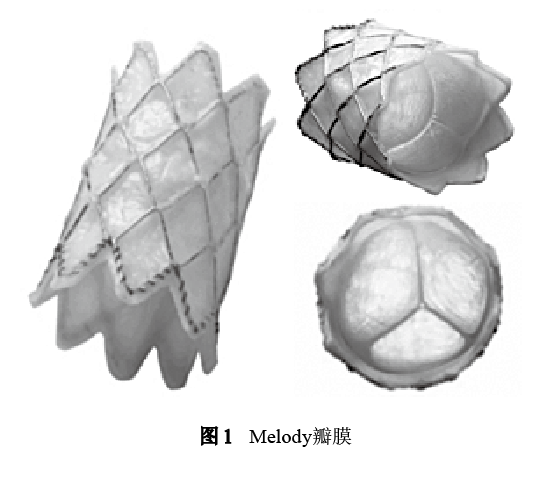
The Ensemble delivery system used by Melody’s pulmonary valve includes Tip, inner and outer balloons, traction guidewire tube/inner balloon tube/outer balloon tube system, outer sheath, compatible guidewire, guide sheath, hemostatic valve, etc. The outer diameter of the distal end is 22 F, and the outer diameter of the proximal end is 15 F. The outside is a retractable polytetrafluoroethylene (PTFE) outer sheath, which is usually delivered to the pulmonary artery with a longer introducer sheath of larger size. It is recommended for body weight> 20 kg patient. The balloon adopts the Balloon-in-Balloon high-pressure double balloon expansion system. The inner balloon diameter is half the diameter of the outer balloon during expansion. The balloon is made of nylon. The outer balloon diameter is 18 mm, 20 mm, and 22 mm. (24 mm is not approved), the valve must be manually crimped onto the balloon before insertion.
At present, the long-term complications (including stent rupture, infective endocarditis, etc.) and long-term efficacy data of TPVR abroad are almost all based on the results of clinical research on the Melody valve. 76% of patients with Melody valve do not need to intervene in 5 years, and the improvement of long-term cardiac function in patients with stenosis and reflux is more obvious than that in patients with only reflux. There is still a lack of data on the results of valve durability and the rate of exemption from reintervention for more than 5 years.
The most common reason for re-intervention is stent fracture leading to restenosis of the implanted valve, and 15% of the causes are implant removal and valve replacement caused by infective endocarditis. Existing evidence indicates that Melody valve stent fracture often occurs in patients with dysfunction of the pipeline or autologous outflow tract valve, which is likely to be caused by repeated stress on the platinum-iridium alloy stent. The incidence of stent fracture in the literature is 5%~25%. Risk factors include younger age, higher preoperative and postoperative transvalvular pressure difference, small diameter of the tube, recoil or incomplete expansion of the stent after release, and close proximity of the valve Directly below the breastbone, large pipe curvature, etc.
Nordmeyer et al. classified stent fractures: most of them are type I stent fractures, at least one stent column fractures, without loss of stent integrity, which is often observed in the early postoperative period, has little clinical significance, and is usually not related to adverse events. No further intervention is required, but its progress needs to be monitored regularly. Type II (stent fracture with loss of stent integrity) and Type III (stent fracture with separation of stent fragments or distal embolization) usually require re-intervention because it can lead to tube narrowing and Valve dysfunction.
Pre-insertion of a balloon-expandable bare metal stent as a landing area before the Melody valve implantation can significantly reduce the incidence of stent fracture, while ensuring the morphological stability of the valve stent and postoperative hemodynamic indicators. Reduce the probability of re-intervention. Adequate post-expansion after valve placement is also believed to reduce stent fracture and related restenosis. Transcatheter valve implantation is the main method to treat Melody valve stent fracture.
2. 2 Sapien valve
The Sapien series was first used for transcatheter aortic valve implantation. In 2006, the first human implantation of a pulmonary valve was implemented in the United States. In 2010, it obtained the CE Mark. In 2016, Sapien XT was approved by the FDA for the use of transcatheter pulmonary valve for artificial tube failure. Put in. The Sapien XT valve has three sizes of 23 mm, 26 mm, and 29 mm, and the corresponding stent heights are 14.3 mm, 17.2 mm and 19.1 mm. The stent material is a cobalt-chromium alloy formed by laser cutting, and the leaflets are bovine pericardium, stitched on the inside of the stent, and stitched with a sealed skirt of PET material (Figure 2).
The third-generation Sapien 3 valve (models 20 mm, 23 mm, 26 mm, 29 mm, corresponding to stent heights of 16 mm, 18 mm, 20 mm, and 22.5 mm) has been commercialized in the aortic position and is currently undergoing clinical TPVR The trial evaluated its effectiveness (COMPASSION S3 Clinical Trial nct0274677). In addition to valve failure, it has also been successfully used for bioprosthetic valve implantation and autologous outflow tract implantation. Due to its larger size, it can be placed in a larger diameter outflow tract. Some scholars have also tried to use the new soft Nucleus balloon delivery system to expand the Sapien XT valve to a larger size.
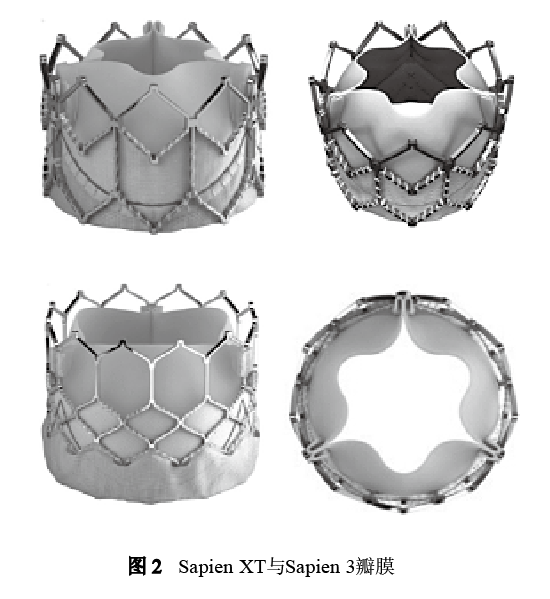
Sapien XT valve delivery system adopts transfemoral Nova Flex system/transventricular Ascendra system (Sapien 3 valve is Commander system, the size is reduced to 14~16 F), the size is 16~20 F, Nova Flex system includes 2 catheters (balloons) Inner catheter and outer balloon catheter), start the valve at the proximal end of the outer catheter delivery rod balloon, withdraw the balloon catheter when passing through the inferior vena cava/abdominal aorta, keep the position of the outer catheter unchanged, and make the balloon approach the valve .
According to the X-ray markings at both ends of the balloon, the valve placement site can be optimized. The valve is expanded using a volume-controlled single balloon (expansion volume is 17 mL, 22 mL, 33 mL, and burst pressure 7 atm), and is pre-loaded on the conveyor balloon without protection of the outer sheath during delivery. The conveying handle has a bending system. For larger autologous RVOT, some people have tried to place multiple overlapping stents in the outflow tract to create a suitable landing area for the transcatheter valve.
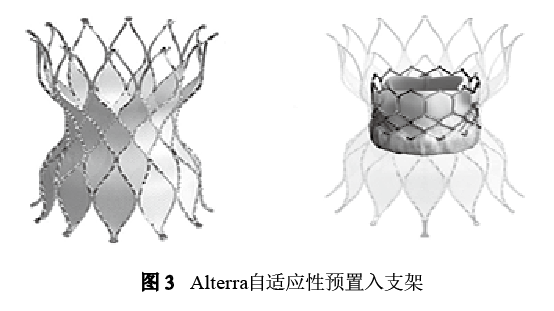
For those with larger RVOT-pulmonary artery diameter, some scholars have tried to insert a Sapien valve into each of the left and right pulmonary artery branches. This kind of different position implantation also has a good effect, and dozens of cases have been reported, but the technical difficulty is not easy. The failure rate and death rate are high. In order to adapt to a larger diameter autologous outflow tract, Edwards developed a percutaneously implanted Alterra adaptive pre-inserted stent in 2018, which aims to reshape the autologous RVOT internally, reduce the RVOT diameter and create a rigid landing zone. After docking the adapter, a standard 29 mm balloon expandable valve was inserted. The first human implantation test has been completed.
The stent is a symmetrical hourglass design. Except for the outflow end, most of the stent is sutured with PET film on the inner surface of the stent. The outflow mesh is non-covered. The inflow and outflow diameter is 40 mm, the center diameter is 27 mm, and the total length is 48. mm, the length of the fully covered part is 30 mm, and it is inserted into the autologous outflow tract through 16 Fr eSheath (including the retractable outer delivery sheath, the inner delivery sheath of the stent, the connector, the tapered tip, the handle, the flush port, etc.) It was then placed with a 29 mm Sapian 3 valve (Figure 3). Currently conduct an early feasibility study in US.
Judging from the results of current clinical studies, it is rare that the Sapien valve stent fractures, whether or not the stent is pre-inserted, which may be related to the more durable cobalt-chromium stent. The latest report of the COMPASSION study showed that the rate of avoidance of re-surgery 4 years after TPVR operation was 91.8%, the rate of avoiding re-operation of TPVR 4 years after operation was 91.2%, and there was no surgery-related death or stent 5 years after operation. The fracture occurs.
3. Features and limitations of self-expanding valves
Reconstruction of RVOT using transvalvular annulus patch technology is very common in correction surgery for tetralogy of Fallot, but it can lead to significant RVOT-pulmonary artery dilation and disused PR in the long term. The size of RVOT can easily exceed the currently available ball The maximum size of the balloon-expandable valve, so it is not suitable for balloon-expandable valves in most cases.
The current size of the transcatheter pulmonary valve is mainly determined by the size of its own valve annulus. It has been reported that the insertion of a transcatheter pulmonary valve in a patch-enlarged autologous RVOT requires an insertion site with a diameter of less than 29 mm, so approximately two-thirds of patients with extra-large pulmonary annulus are excluded from the percutaneous treatment program. Another report estimated that only 15% of patients with autologous outflow tract PR are eligible for transcatheter balloon dilatation valve placement.
There is an urgent clinical need for transcatheter pulmonary valve devices dedicated to dilated autologous RVOT. At present, at least 4 valves are undergoing preliminary clinical studies to try to fill this gap. However, unlike the right ventricular pulmonary artery duct, the anatomy of autologous RVOT is very complicated. It is a big challenge to design and insert a self-expanding system that can adapt to various autologous RVOT anatomy after surgery.
Superelastic Nitinol may be the most suitable The material of the stent is high, so that the stent has a large reversible deformation ability and anti-fatigue and anti-fracture ability in the whole heart cycle. The overall structure of the existing products is similar. Most of the released stents are introduced into one branch of the pulmonary artery, and the distal end is slowly released at the level of the branch opening until the valve is fully deployed at the distal end of the main pulmonary artery (sometimes it is necessary to release the stent. Pull the distal end back to the location of the main pulmonary artery where it is expected to deploy), and then release all the stents.
3. 1 Harmony valve
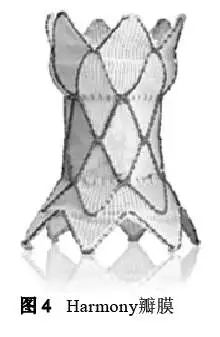
In 2010, Schievano et al. reported for the first time that a self-expanding percutaneous pulmonary valve was placed in the expanded autologous outflow tract, which was later named the Medtronic Harmony valve for the treatment of autologous outflow tract PR. The preoperative image evaluation of patients used data calculation and simulation models created by four-dimensional CT, and improved the main screening methods for patients in subsequent studies.
The Harmony valve is dumbbell-shaped and has a larger diameter at the proximal and distal ends. The porcine pericardial valve is located in the narrow part of the center (Figure 4). Depending on the temperature-dependent shape memory characteristics of Nitinol, it is released into different outflow tracts. In the anatomical structure. The porcine pericardium tissue was sterilized with α-aminooleic acid anti-calcification process and 0.2% glutaraldehyde.
The valve delivery system is a 25 Fr coil loading system and an integrated catheter sheath. An early feasibility study reported that 20 patients were successfully implanted and completed a 2-year follow-up. Except for 2 patients who required early surgical instrument removal (1 case within 24 hours) Distal displacement and 1 case of proximal type II stent fracture caused obstruction), the stent integrity and valve function of the remaining 18 cases were maintained well, the average pressure gradient was (15±6) mmHg, and 2 cases had mild paravalvular leakage. Three cases had type I stent fracture.
The valve used in the early study was only a single model. The diameters of the distal corolla, waist, and proximal corolla were 34 mm, 23.5 mm, and 42 mm, respectively, and the total length of the stent was 55 mm. The preliminary results of the study have proved the long-term effectiveness and safety, but also suggest that the detailed design of the product and the expansion of the model need to be improved to solve some of the problems that have been discovered. The Harmony valve is currently undergoing a phase III multi-center clinical trial in the United States (Medtronic Harmony TPV clinical trial NCT 02979587), and more specifications and models have been designed.
3. 2 Venus P valve
Venus P Valve is a self-expanding, hourglass-type transcatheter pulmonary valve developed by Hangzhou Qiming Company, which is used for percutaneous insertion into the right ventricular outflow tract of the body. The first implantation was completed in Shanghai Zhongshan Hospital in 2013. At present, 55 implants have been completed in 6 centers in domestic clinical trials and are waiting for pre-market approval. Venus P has completed more than 220 implants in 27 centers in 16 countries around the world, and has completed the CE mark clinical study enrollment (80 cases), and the FDA approval IDE study will be launched soon.
Venus P valve stent consists of 3 parts, both ends are flared, the distal flared is a completely hollowed bare metal stent, the mesh is large and soft to avoid blocking and damage to the branch blood flow, the lower horn and the middle straight section The covered porcine pericardium tissue is sutured and fixed on a nickel-titanium alloy stent. The three-leaf porcine pericardial valve is sutured on a porcine pericardium skirt with polyethylene sutures.
The middle straight section where the valve is located is longer, and the main Support the riveted part (Figure 5). According to the diameter of the middle segment, the valve model ranges from 16 mm to 32 mm. Each 2 mm is a specification, and there are different radial lengths of the middle segment. The diameter of the proximal inflow channel is 10 mm larger than that of the middle section, and the distal outflow channel has a larger diameter. The middle section is 7 mm larger. Based on previous experience, the valve model selection criterion is to add 2~4 mm to the diameter of the pulmonary valve annulus.
Due to the good compliance characteristics of the autologous outflow tract, the valve annulus diameter is mainly obtained by intraoperative balloon measurement. Early research data showed excellent hemodynamic results and improved right ventricular volume. At the same time, Venus P has developed larger valve sizes of 34 mm and 36 mm to enable more patients to accept this operation.
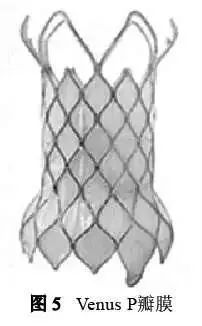
The Venus P valve catheter delivery system includes a catheter handle, a pusher, a sheath, a capsule cavity, and a tip. The middle rod of the catheter is 100 cm in length to ensure that the valve can reach the release position and provide steering force and torsion control force. The artificial pulmonary valve is manually loaded in the head capsule cavity outside the body, and the catheter handle is used to release the valve after accurate positioning after being introduced into the body. The valve can be released by rotating the fine adjustment knob on the handle. The main catheter of the delivery system is 16 F, and the capsule cavity of the valve is 20~22 F. There is a set of X-ray opaque Marks at the intersection of the valve position, the middle straight section of the stent and the horns at both ends, which can be inserted into the valve. Monitor valve release and determine location during the process.
3. 3 Pulsta valve
The Pulsta valve invented in South Korea is another self-expanding transcatheter pulmonary valve designed for autologous RVOT. The stent shape is closer to a straight cylindrical shape. The skeleton is a double-strand braided nickel-titanium alloy wire with a diameter of 0.0115 in (1 in=2.45 cm). , And there is no welding (Figure 6). Compared with other self-expanding valves, the stent is softer, and has a better potential to prevent stent fracture in autologous RVOT lesions with large compliance and high diameter change rate, while reducing the size of the delivery system.
The waist of the stent is slightly curved and concave, and the maximum diameter of the upper and lower ends is only 4 mm larger than the waist diameter. The waist of the valve is 18-28 mm in diameter and 28-38 mm in length. In vitro testing of larger 30 mm and 32 mm valves has been completed and is expected to be used in clinical practice soon. The Pulsta valve undergoes a multi-step special processing process to maximize tissue retention, reduce calcification, reduce immunogenicity, and prevent early degeneration.
The procedures include decellularization, α-galactosidase treatment to remove α-galactose xenoantigens, Space filling treatment, glutaraldehyde fixation, organic solvent treatment, and detoxification are the first porcine pericardial valve without α-galactose. After treatment, more than 400,000 stent fatigue tests and more than 200 million valve accelerated wear tests were completed. The inner layer of the stent is covered with porcine pericardium tissue and 5-0 polyester braided silk is tightly hand sutured to the stent wall to ensure that the trimmed tri-leaf porcine pericardial valve is well aligned after suture. Most of the stent walls except for the two ends are covered with porcine heart.

The transcatheter delivery system for the Pulsta valve has a usable length of 110 cm, the tapered tip length is 17 mm, the valve loading cavity is 18 F, and the delivery catheter shaft is 12 F. A hook block is designed at the hinge joint between the delivery system and the proximal end of the valve, which can control the stable release of the valve and effectively prevent the valve from jumping out suddenly during the release process. According to reports, 10 human implants have been successfully completed, and a multi-center clinical trial in South Korea is currently underway to further evaluate safety and effectiveness (clinical trials.gov identifier: NCT03110861).
3. 4 PT Valve valve
The domestic PT Valve has completed more than 20 human implants in China. Early feasibility studies have shown good safety and effectiveness. It is characterized by its dumbbell-shaped, symmetrical nickel-titanium alloy stent design. The ends of the stent are soft and flexible. The stent at the lumbar valve position is small and hard. The connection between the outflow end of the stent and the waist is an open-loop design.
The valve is cut and sutured from porcine pericardial valve leaflets, and the porcine pericardium sealing membrane is inside the stent (Figure 7). According to the diameter of the corolla and waist of the stent, valve models: 28 mm-20 mm, 32 mm-23 mm, 36 mm-26 mm, 40 mm-26 mm, 44 mm-26 mm, and there are 5 stent sizes And 3 valve sizes, of which the 26 mm valve has 3 different frames (36 mm/40 mm/44 mm). In the future, two other sizes of 48 mm-29 mm and 52 mm-29 mm are also being developed to more adapt to the anatomical characteristics of the autologous outflow tract of the Chinese. The capsule cavity of the delivery system is 21 F, the push rod part is 12 F, and the tail end has a movable hemostatic valve design.
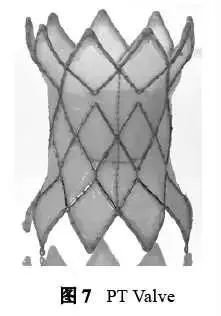
The positioning of the valvular stent mainly relies on the swelled corollas on both sides being riveted in the main pulmonary artery and outflow tract (multi-planar measurement is required before surgery). The model selection is not limited by the diameter of the valve annulus, so it can handle the diameter of the lung annulus exceeding 30 mm. Patients (up to 36~38 mm).
Because the relatively narrow center of the stent is not compressed at the lumbar valve position, it does not affect the performance of the valve leaflets, reduces the increase in transvalvular gradient and mechanical shear force caused by incomplete leaflet deployment, does not affect the durability of the valve, and can significantly reduce the stent at the same time The risk of coronary artery compression caused by external pressure in the middle segment (the left coronary artery often runs near the left posterior of the valve annulus), and most cases do not even need to perform a balloon compression test. The stent porcine pericardial full membrane design makes the stent and the outflow tract have enough contact surface to reduce the occurrence of paravalvular leakage.
4. Surgical techniques and clinical issues
There may be technical difficulties in TPVR surgery. In view of the patient’s complex outflow tract anatomy, it is very challenging to push a large valve delivery system to the distal end of the pulmonary artery. A variety of manipulation techniques, including manual adjustment, bilateral double guide wire orbit, RVOT auxiliary balloon, snare stretching and bending, right atrium recoil support, and transjugular route can all be tried.
The rail wire used for delivery generally can use the Lunderquist extra-hard guide wire, and it needs to be pre-shaped into a pigtail curve to protect the pulmonary artery branches and prevent damage to the distal end caused by the pushing process and breathing movement. In cases of stenosis and calcification, Lunderquist may be too hard and needs to be replaced with a softer Amlplatz Superstiff guide wire. The incidence of complication of duct tear or rupture caused by violent operations in the current study is 4.1%.
However, due to extensive local adhesions after surgery, most of them are local oozing and do not cause hemodynamic abnormalities. Dissection or rupture of the distal pulmonary artery in some cases may be life-threatening and requires stent graft or surgical treatment as soon as possible. Some foreign centers have statistics on the failure rate of valve implantation technology, transfer to surgery, and transthoracic insertion of the valve. The incidence rate can be as high as 10%. The selection of intraoperative guidewire and delivery technology and the accumulation of experience are through repeated Experiment to achieve.
The TPV model selection strategy mainly depends on the native valve annulus diameter or the narrowest diameter of the main pulmonary artery, that is, relying on the support force generated by the compression of the stent at the valve level to ensure safe placement, or relying on the effective riveting of both ends of the stent to ensure the stability of the device. Even so, because the diameter of the pulmonary valve annulus in most patients has actually exceeded 30-40 mm, it obviously limits the choice of TPV valve models and anatomical indications.
According to the current clinical selection experience of many self-expanding valves, only 20%-40% may meet the anatomical indications of TPVR. The choice of valve model is too small or the anatomical indications are inappropriate, which are generally related to the surgical planning, release strategy, indication selection and measurement error of the pulmonary annulus diameter. Especially for inexperienced centers, severe valve displacement can cause catastrophic consequences, and it is necessary to switch to surgical thoracotomy as soon as possible. Balloon-expanded valves with short stents are more likely to be displaced and embolized distally, which can lead to pulmonary artery obstruction and usually require surgical removal.
The self-expanding valve with long stent is more susceptible to downward displacement under the influence of pulmonary artery pressure. Because the expanded pulmonary artery and expanded outflow tract patch are very compliant, the diameter varies greatly with the cardiac cycle, can it be used as a riveting site, In addition to the maximum diameter during systole, the overall length and curvature of RVOT should also be combined. The valve can enter the right ventricle completely when the valve is displaced downwards. Therefore, for patients with the anatomical features of the autologous outflow tract with an inverted funnel and large diameter, the surgical selection should be very careful.
Due to the variability of coronary artery anatomy in patients with complicated congenital heart disease, it was found that 5% to 7% of patients were at risk of coronary artery compression in the autologous RVOT/tube balloon compression test. Since the left main coronary artery usually runs on the left posterior near the level of the pulmonary valve annulus, TPVR cannot be implemented in most cases for those with greater risk. Considering the incidence of this catastrophic complication, a detailed assessment of its anatomical characteristics and potential compression risk is required.
So far, several cases of coronary artery occlusion and death caused by compression have been reported in the literature, and there are even coronary artery compression that occurred several months later after surgery. The current standard method for detecting the risk of coronary artery compression is to perform a compression test with a balloon of the same size as the planned valve. The current guidelines for TPVR surgery all recommend a coronary artery compression test (grade Ib recommendation). Preoperative computed tomography angiography (CTA) evaluation is also very important in identifying high-risk groups.
Three-dimensional rotational angiography, CTA and digital subtraction angiography (DSA) fusion imaging are now also used in coronary artery risk assessment. In addition to coronary artery compression, there have also been reports showing that it can cause significant aortic root compression and aortic regurgitation in balloon compression tests. PT Valve and Harmony Valve, due to their narrow waist design and non-supporting riveting method at the waist, theoretically may not cause compression on the annulus and peripheral tissues, and can reduce the risk of coronary artery compression.
Limited literature reports suggest that preoperative CTA and careful planning of surgery to conduct a detailed assessment of coronary artery risk, it is possible that most of the coronary artery compression tests that must be performed during the valve placement process can be omitted, and the surgical process is simplified, but it is still not enough to change the current guidelines and recommendations, especially for Cases with pulmonary valve stenosis.
5. Outlook
Trans-ring patch is widely used in domestic correction surgery for tetralogy of Fallot (it is estimated that more than 85%). In recent years, more than 10,000 operations have been performed each year. Most of these patients may face a large number of surgeries in 10 to 30 years. PR requires a second operation of the pulmonary valve, and the number of patients in the future may face a blowout. Transcatheter pulmonary valve technology has been proven to be a safe and effective method for the treatment of PR.
Patients with severe PR after correction of tetralogy of Fallot with suitable anatomical structure have become an alternative to surgical PVR and can be used as the first choice for treatment. . However, even when a variety of balloon dilatation and self-expanding valve products are available, only 20%-40% of patients meet the anatomical indications of TPVR. Those with an inverted funnel-shaped outflow tract with insufficient distal length and an outflow tract with a full diameter greater than 40 mm are still considered unsuitable for TPVR. A TPV suitable for these two outflow tract anatomical characteristics has been developed Can benefit more patients.
The development of a transcatheter valve with a smaller size and softer delivery device can enable lower-age and low-weight patients to have the opportunity to undergo TPVR surgery, while ensuring more accurate implantation, and also conducive to the retrievability, repositioning or migration of the valve. except. It is also one of the necessary trends to develop self-expanding valves with larger diameters and shorter lengths to expand anatomical indications. The RVOT remodeling device is expected to expand the indications for the placement of the ball-expandable valve. The hybrid operation of surgical thoracotomy RVOT/pulmonary artery folding and TPVR can solve some patients with excessively wide outflow tract structure and high-risk coronary artery risk.
At present, TPVR products still have complications and risks such as device displacement, paravalvular leakage, infective endocarditis, stent rupture, restenosis or coronary artery obstruction, which reduces complications that require re-intervention, and increases the clinical effect and long-term durability of the device In the future, new valve products also need to focus on these issues.
Research on emerging technologies such as cardiac magnetic resonance analysis and three-dimensional CT fusion imaging will help to better determine the timing of surgical intervention and simplify the difficulty of surgical operations. Because the patient is relatively young, the long-term durability of the transcatheter pulmonary valve is also worthy of attention.
Valve life is largely limited by progressive valve degradation secondary to host immune response and dystrophic calcification. In preclinical studies, it has been demonstrated that autologous stem cells have been used to successfully develop and implant tissue engineered pulmonary valves.
Advances in tissue engineering valves and adaptive material design can enable pediatric prosthetic valves to adapt to the expansion of the heart structure as children age, and to maximize valve service life. In view of the current trend of early intervention, the use of TPVR may be expanded in the pediatric patient population in the future.
New technology for transcatheter pulmonary valve replacement
New technology for transcatheter pulmonary valve replacement
New technology for transcatheter pulmonary valve replacement
(source:internet, reference only)
Disclaimer of medicaltrend.org



