Innovation and progress of transcatheter pulmonary valve replacement
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Innovation and progress of transcatheter pulmonary valve replacement
Innovation and progress of transcatheter pulmonary valve replacement. Transcatheter pulmonary valve replacement (TPVR), also known as Percutaneous pulmonary valve implantation (PPVI), was first reported by Professor Philipp Bonhoeffer in 2000 [1], mainly used in right Patients with right ventricular outflow tract dysfunction (including stenosis and regurgitation) after ventricular outflow tract reconstruction, the most common of which is patients after surgical correction of Tetralogy of Fallot (TOF).
At present, more than 10,000 patients worldwide have undergone TPVR treatment. Since the completion of the first TPVR in some countries in 2013[2], many centers have successively carried out and completed nearly a hundred operations, in terms of technical specifications and device innovation. Made innovation and progress.
1. Indications of TPVR
Congenital heart disease with stenosis of the right ventricular outflow tract requires reconstruction of the right ventricular outflow tract during surgical correction. Foreign surgeons often use the method of implanting valved vascular channels. As patients age, valved vessels The channel may have restenosis and valve regurgitation. In China, the right ventricular outflow tract-pulmonary artery transvalvular patch is mostly used, and pulmonary regurgitation will be left after the operation.
Long-term severe right ventricular outflow tract dysfunction can lead to right heart failure and even total heart failure, significantly shortening the patient’s survival period [3-4]. Therefore, patients with right ventricular outflow tract dysfunction and right heart insufficiency need intervention treatment, the main methods include Surgical pulmonary valve replacement (SPVR) and TPVR.
2018 AHA/ACC Adult Conscience Guide
The 2018 AHA/ACC Adult Congenital Heart Disease Management Guidelines [5] recommends symptomatic patients with moderate or higher pulmonary regurgitation after TOF correction (Class I), or asymptomatic patients with significant right ventricular enlargement and decreased systolic function (Class IIa) Surgery or transcatheter pulmonary valve replacement. However, it does not give any recommendations on the preferred surgical procedure. Later studies compared the clinical outcomes of SPVR and TPVR at 30 days, 1 year and 3 years after surgery. The results showed that there was no significant difference in death, cardiovascular rehospitalization, and pulmonary valve re-intervention rates between the two, but 30 days after TPVR The incidence of right heart dysfunction is significantly less than that of SPVR, even if the preoperative baseline heart function of TPVR patients is worse [6]. It can be seen that TPVR has the advantages of less trauma, faster recovery and lower surgical risk than SPVR.
2020 ESC Adult Guide
The 2020 ESC Guidelines for the Management of Adult Congenital Heart Disease[7] suggest that patients with symptoms and severe pulmonary regurgitation and/or at least moderate right ventricular outflow tract obstruction are recommended to undergo SPVR or TPVR (Class I), asymptomatic and severe pulmonary artery Patients with valve regurgitation and/or right ventricular outflow tract obstruction should have one of the following criteria (objective exercise performance index decline; right ventricular progressive expansion to end-systolic right ventricular volume index ≥80mL/m2, and/or end-diastolic right Ventricular volume index ≥160mL/m2, and/or tricuspid regurgitation progresses progressively to at least moderate; right ventricular systolic function progressively decreases; right ventricular outflow tract obstruction combined with right ventricular systolic pressure> 80mmHg) should consider SPVR or TPVR (Class IIa) (Figure 1).
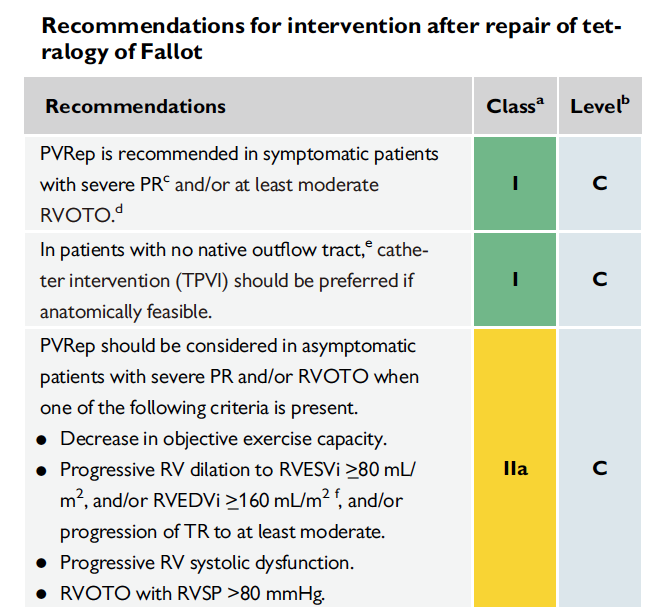
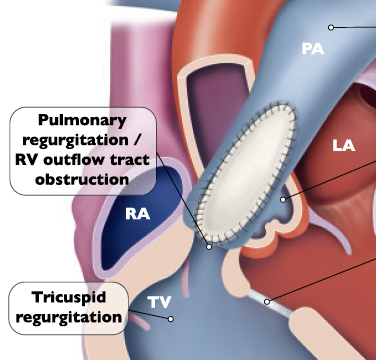
Figure 1: 2020 ESC Adult Guideline
Suggestions on the occurrence of pulmonary regurgitation after the fourth method
At the same time, the guidelines clearly point out that for patients without congenital outflow tracts (including patients who have previously used allogeneic, porcine jugular vein grafts, biological valves/catheters for RVOT surgery), TPVR (Class I) is recommended if anatomical conditions are feasible. ).
2016 China recommendations for percutaneous pulmonary valve placement
In 2016, Chinese experts recommended percutaneous pulmonary valve placement [8] and put forward specific indications and contraindications for TPVR.
Indications include:
(1) Moderate to severe pulmonary regurgitation after surgical correction of congenital heart disease with right ventricular outflow tract stenosis.
(2) The patient has symptoms related to right ventricular outflow tract dysfunction, including impaired exercise tolerance, right heart failure: or the patient is asymptomatic but has any of the following conditions (①moderate or above functional tricuspid regurgitation; ②heart Right ventricular end-diastolic volume index measured by magnetic resonance imaging ≥130 mL/m2; ③Right ventricular ejection fraction measured by cardiac magnetic resonance imaging <45%; QRS wave width ≥160 ms; persistent atrial or ventricular rhythm Abnormal).
(3) Anatomically suitable for TPVR.
(4) Age ≥ 10 years old or weight ≥ 25 kg.
Contraindications include:
(1) Pulmonary hypertension (mean pressure ≥ 25 mmHg).
(2) Severe pulmonary artery or branch stenosis.
(3) The anatomical evaluation is not suitable, including the inability of the valve to enter the valve or the right ventricular outflow tract-the pulmonary artery cannot place the valve, or the preoperative examination suggests that the valve stent may compress the coronary artery.
(4) There are contraindications for cardiac catheterization.
2. International TPVR valve
Because foreign countries choose valved vascular channels for surgical right ventricular outflow tract reconstruction, balloon-expandable valves are mostly used in TPVR internationally. Because of the support of valved vascular channels, the balloon-expanded valve is easy to anchor, and only part of the outflow tract has Patients who expand need to be implanted with a ball expansion stent in advance to ensure the stability of the valve stent, or use a self-expanding valve.
At present, the international TPVR ball expansion valve system mainly includes Medtronic Melody valve and Edwards Sapien valve, of which the Medtronic Melody valve system is the most widely used.
Medtronic Melody valve is the first valve used for TPVR. It consists of a bovine jugular vein valve and a platinum-iridium alloy stent (Figure 2). It was approved by CE in 2006 for patients with right ventricular outflow tract dysfunction. It was approved by the US FDA in 2017. Batch. Its diameter model is 18mm-22mm. Multi-center clinical trials [9] have proven that its implantation success rate in patients with valved vascular access can be as high as 99%, and it can effectively improve the dysfunction of the right ventricular outflow tract and obtain very excellent early intervention results. According to the results of the mid-term follow-up [10], the 5-year avoidance rate of re-intervention is about 76%. The restenosis caused by the rupture of the valve stent is the main reason for the re-intervention. The pre-implantation of the stent can reduce the re-intervention rate. However, in patients with pulmonary regurgitation without valved vascular access, the success rate of Melody valve implantation is only 58%, mainly due to the over-dilated right ventricular outflow tract.


Figure 2: Medtronic Melody valve (Medtronic, Minneapolis, Minn)
The Edwards Sapien valve is the second balloon-expandable valve, composed of a bovine pericardial valve and a nickel-titanium alloy stent (Figure 3). It was implanted for the first time in the United States in 2006 and received CE approval in 2010. Its diameter model is 20mm-29mm. The early clinical results of a multi-center retrospective observational study of Edwards SAPIEN XT valve showed that the overall implantation success rate was 93.5% in patients with or without valved vascular access, and patients with right ventricular outflow tract dysfunction and clinical practice 30 days after surgery Cardiac function has been significantly improved [12]. The COMPASSION study aimed at the use of Edwards Sapien valve in patients with valved vascular access. The 3-year follow-up results showed that 93.5% of patients had improved clinical cardiac function, and the dysfunction of the right ventricular outflow tract was significantly improved compared with baseline. Among them, 91.1% of patients achieved The rates of pulmonary valve regurgitation below mild, avoiding death, re-intervention and endocarditis were 98.4%, 93.7% and 97.1%. There was no stent fracture. 91% of the patients had pre-stent implantation. [13].


Figure 3: Edwards Sapien valve
(Edwards Lifesciences, Irvine, California)
At present, there is no TPVR self-expanding valve approved by CE or PDA in the world, but there are many types of valves in development, and three of them have entered clinical trials, including Medtronic Harmony valve (20 cases of human implantation have been completed), South Korea SNU-TaeWoo Med’s Pulsta Valve (10 cases of human implantation have been completed) and Edwards’ Alterra Adaptive Prestent valve and bare stent (the first case of human implantation has been completed). As shown in Figure 4.
TPVR



Figure 4: International TPVR self-expandable valve
3. Transcatheter pulmonary valve-in-valve implantation technology (TPV-in-TPV)
The main complications of TPVR mid- and long-term surgery include infective endocarditis, prosthetic valve obstruction, stent rupture, and valve failure. Some patients need re-interventional treatment after TPVR, including balloon dilation, bare stent implantation and TPV- in-TPV. Shabana Shahanavaz et al. [19] included 309 patients who used Melody valves for TPVR in 3 prospective multi-center studies. During the follow-up period, 46 patients underwent re-intervention, and all patients had artificial valve restenosis, 15% With endocarditis, 59% also had valve stent fracture. Among the re-interventional patients, 17 cases underwent simple balloon dilatation (median time after TPVR was 4.9 [4.0-6.0] years), 1 case underwent bare metal stent implantation (4.4 years after TPVR), and 28 cases underwent TPV-in-TPV (median time after TPVR is 6.9 [5.2-7.8] years). Comparing the simple balloon dilatation group and the TPV-in-TPV group, both can significantly improve the hemodynamic parameters (including the mean transvalvular pressure difference of the heart, the peak pressure difference of the right ventricular outflow tract measured by the catheter, and the right ventricular outflow tract). Ventricular systolic pressure, right ventricular/aortic pressure ratio), but the immediate improvement effect of the two is similar. The rate of avoiding re-intervention within 4 years after re-intervention was 60%, and the rate of avoiding transplant was 83%. The rate of patients undergoing TPV-in-TPV avoiding re-intervention was significantly higher than that of patients with simple balloon dilatation (71% vs. 46% at 4 years; p = 0.027), indicating that although balloon dilatation and TPV-in-TPV It can also improve the patient’s immediate hemodynamics, but TPV-in-TPV can more persistently relieve RVOT obstruction and pulmonary regurgitation.
4. Technological Innovation in TPVR
In TPVR, the failure of the valve-loaded delivery system to cross the valve is the main cause of surgical failure, and its incidence is about 2% [20-22].
The main factors that cause straddling difficulties include:
- Right atrioventricular expansion, transposition, tortuous path;
- Right ventricular inflow tract and outflow tract form an angle;
- Calcification and local stenosis of the right ventricular outflow tract;
- The profile of the conveying system is large, the overall is hard, and the passability is poor;
- Insufficient support of steel wire track.
Common solutions include:
- Look for the branch of the pulmonary artery where the guide wire can be inserted, usually the left lower pulmonary artery is the first choice;
- Use the hardest guide wire as the track support, usually lunderquist super hard guide wire;
- Strengthen the support of the auxiliary road, straighten the path across the valve, including the auxiliary road with another super-hard guide wire and hard sheath support
However, in some special cases, conventional solutions cannot solve the cross-valve problem. The team of Academician Ge Junbo and Professor Zhou Daxin created a new cross-valve technology during the live demonstration of the structural week surgery in September 2020, which was named “Support sheath and snare Parallel Anchor-Supporting sheath and Snared wire (PASS), that is: the snare is fed into the support sheath of the auxiliary road, and the main wire track is covered with the snare to firmly grasp the main wire The distal end prevents it from withdrawing due to the forward resistance of the delivery system and loses support, allowing the support sheath and the snared guide wire to form an anchored whole, providing the strongest delivery system support. Coincidentally, in October 2020, Dr. Nicola Maschietto published his experience in successfully performing TPVR using the snared wire technique (SWT) in the Catheter Cardiovasc Interv magazine [23]. PASS and SWT coincide in core technology, fully demonstrating the rich experience of TPVR experts and their passion for maintaining technological innovation.
5. Summary
With the increase in clinical application and evidence of TPVR, the guidelines have fully affirmed its value in patients with right ventricular outflow tract dysfunction (including stenosis and regurgitation) after right ventricular outflow tract reconstruction, and it has become the preferred treatment for patients with anatomically suitable patients. . The Chinese patient population has the unique anatomical features of tumor-like expansion of the right ventricular outflow tract. The self-expanding valve developed in China is adapted to such patients with its unique design. Pre-clinical trials have obtained good results in terms of safety and effectiveness. However, there is a certain incidence of long-term valve dysfunction. TPV-in-TPV can more persistently relieve the recurring right ventricular outflow tract obstruction and pulmonary regurgitation, and may be the first choice for TPVR re-intervention.
We believe that with the maturity and perfection of TPVR technology, the development and improvement of valve devices will improve the success rate of surgery, reduce mid- and long-term complications, and increase valve durability, etc., will be overcome one by one.
(sourcechinanet, reference only)
Disclaimer of medicaltrend.org



