How to optimize the medium design to enhance CAR-T cells?
- Why Lecanemab’s Adoption Faces an Uphill Battle in US?
- Yogurt and High LDL Cholesterol: Can You Still Enjoy It?
- WHO Releases Global Influenza Vaccine Market Study in 2024
- HIV Infections Linked to Unlicensed Spa’s Vampire Facial Treatments
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
How to optimize the medium design to enhance the anti-cancer function of CAR-T cells?
How to optimize the medium design to enhance the anti-cancer function of CAR-T cells? Adoptive immunotherapy provides a promising method for the treatment of a variety of diseases including cancer and chronic viral infections.
In these therapies, synthetic receptors are often used to change the specificity of T cells against different cancer antigens. T cells are modified with transgenes to enhance their functions and expanded in a highly controlled ex vivo environment.
In the context of chimeric antigen receptor (CAR) therapy, patient-derived T cells are activated under nutrient-rich conditions, genetically modified and expanded within 9-11 days [1]. An overlooked aspect of the in vitro process is understanding how the metabolic composition of the medium affects the functional properties and behavior of the final cell product.
Activated T cells transform their metabolism from oxidative phosphorylation to aerobic glycolysis to meet the increasing demand for biosynthesis. The anabolic effects of intracellular metabolites and their derivatives in T cell proliferation and differentiation have been well described [2]. Given that activated T cells synthesize macromolecules de novo, including nucleotides, lipids and proteins, they rely on exogenous circulating nutrients. Because the external factors in the microenvironment affect proliferation, phenotype and gene transduction, the overall result of the in vitro culture process is highly dependent on the characteristics of the culture medium.
Adjust the T cell culture medium with serum from animal or human sources. Serum provides an important source of important biologically active peptides, hormones and growth factors. These peptides, hormones and growth factors work together to support cell growth. When designing optimized media for T cell therapy, it is important to understand how serum components affect transduction, proliferation, and differentiation.
Determining the key factors affecting T cell function may lead to the development of chemical derivatives and their inclusion in definite media formulations as conditioning agents. Animal-derived serum, such as fetal bovine serum (FBS), is widely used as a source of nutrition and is widely used in research applications and preclinical discoveries [3].
However, in vitro cell culture with FBS cannot simulate the human microenvironment. This limits the translation application of FBS and emphasizes the need for effective alternatives. FBS is not yet suitable for cell-based therapy because it carries the risk of spreading bovine spongiform encephalopathy and viral pathogens. As a conditioning agent for human cells grown in vitro in a highly controlled environment, human serum (HS) has inherent advantages over cattle.
HS can provide additional stimulation to promote cell proliferation and survival without the need for any foreign ingredients. However, higher serum concentrations can inhibit cell growth [4]. In addition, its limited supply and batch differences will eventually hinder the development of CAR-T cell-based treatments. The metabolic composition of serum changes in a species-specific manner.
For example, uric acid is a metabolic end product that inhibits nucleotide biosynthesis, and its content in humans is 10 times lower than that of mouse or bovine serum [5].
We describe a novel system for transducing and stimulating CAR-T cells using a serum-free method. We show that concentrated growth factor extracts purified from human transfusion grade whole blood fractions can effectively replace serum in CAR-T cell cultures. We found that in the medium conditioned on the concentrated growth factor extract relative to HS, the transduction of CAR-T cells was significantly enhanced. Anti-GD2 CAR-T cells expanded in medium supplemented with Phx showed higher cytotoxicity in vitro.
This helps increase the therapeutic efficacy of neuroblastoma xenograft models. We provide evidence that CAR-T cells expanded in Phx also have excellent implantability and survival rate after transplantation in vivo. Since in vitro culture is the basis of many adoptive immunotherapies, the development of customized serum-free preparations, whether with or without chemically defined derivatives, can protect the biological activity and function of cells, which will lead to the rapid development of CAR-T therapy.
Test results
Several strategies have been developed to increase the anti-cancer efficacy of CAR-T cells. Modifying the CAR design, limiting differentiation and simplifying the manufacturing process have all increased the clinical impact of CAR-T cells. Optimizing media formulations to enhance potency to support the expansion of CAR-T cells is a simple and untested method. In this study, the unique composition of systemic and metabolic factors will enhance the effectiveness of CAR-T cells.
Activated T cells form large blastocysts, ready for cell division. In this primitive stage, T cells synthesize and accumulate the macromolecular components of their daughter cells. To test the effect of Phx on T cell activation and proliferation, we activated T cells with anti-CD3/CD28 Dynabeads in a standard concentration of 5% HS or 2% Phx. We selected a growth factor extract with a final concentration of 2% based on the previously described use in mesenchymal stem cells and CD4+ T cells [18].
As shown in Figure 1A, the average cell volume (representative of activation) between T cells is similar. Stimulate in a standard concentration medium containing 5% HS or 2% Phx. The cell size at 5 days after activation was similar in all groups. The measured values are 705±23 fL (OpT+HS), 719±34 fL (OpT + Phx), 711±26 fL.
After direct or alternative antigen stimulation, activated T cells enter the logarithmic expansion stage. In this proliferation phase, T cells multiply to increase their numbers before adaptive cell transfer. We rigorously tested the effect of Phx on T cell proliferation and survival.
We found that when the clinical grade medium OpTmizer or X-Vivo-15 medium was formulated into 2% Phx or 5% HS, primary human T cells showed similar proliferation ability (Figure 1B). In OpTmizer medium supplemented with 2% Phx, the average population doubling of each cell in 9-11 days was 5.8±0.8. T cells expanded in OpTmizer+5% HS showed an average population doubling of 5.7±0.5. Similar findings were also obtained in X-Vivo-15 medium supplemented with 2% Phx or 5% HS (5.3±0.6 and 5.3±0.7, respectively). The same trend was observed in CD4+ and CD8+ T cell subpopulations.
When 2% Phx (maximum population multiple of 4.7±0.3) is used in RPMI medium instead of 10% FBS (maximum 8.2 times and 1.1 maximum population multiple), T cell proliferation will be moderately reduced. Although the duration of the logarithmic growth phase is not affected, the overall proliferation is reduced (Figure 1C). This effect can be mitigated by increasing the total protein content of the medium with HS albumin (data not shown).
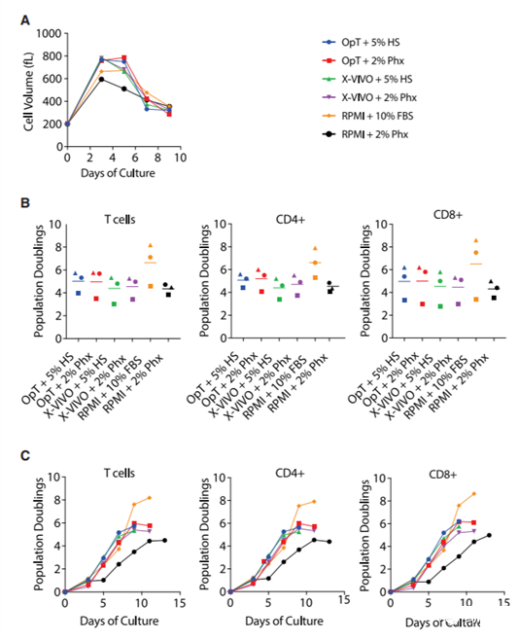
Figure 1. Concentrated blood cell extract Physiologix supports the expansion of primary human T cells in clinical and research-grade media
(A) Stimulate the mixed T cell population with anti-CD3/CD28 Dynabeads, and expand human serum or 2% Physiologix in a medium containing 5% condition. The average cell volume was measured every other day from day 3 until the number of cells in the culture stopped increasing and the average cell volume was below 350 fL. Representative data from 3 independent experiments are shown. (B) Stimulate T cells like (A). Counting cells was performed every other day from day 3 until the number of cells in the culture stopped increasing and the average cell volume was less than 350 fL. The maximum population doubling number is plotted, where the horizontal bar represents the average value, and each symbol represents an individual donor. The data was analyzed by one-way analysis of variance and Neuman-Keuls multiple comparison test. (C) Stimulate T cells like (A). Enumerate the cells at the specified time point. The data for bulk T cells and CD4+ and CD8+ T cell subpopulations represent independent donors in 3 independent experiments.
Since the CD4/CD8 ratio is an important aspect of T cell phenotype, we evaluated the in vitro expansion of a mixed population of CD4+ and CD8+ T peripheral blood T cells. To check whether the proliferation in the CD4 or CD8 region is differentially regulated, we used bead-based flow cytometry to evaluate the surface phenotype of the cells before expansion, during expansion, and at the end of logarithmic expansion. Table S1 shows that the ratio of CD4 to CD8 T cells is comparable between the stimulation conditions. Cell culture models can also provide information about the state of differentiation. We show that at the end of the in vitro culture process, T cells expanded in medium supplemented with FBS, HS or Phx have similar levels of central memory and effector memory surface markers (Figure 2A–2D).
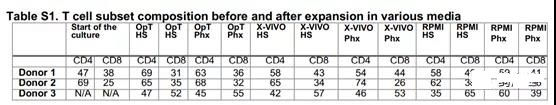
Figure 2. T cells expanded in Phx-conditioned medium are similar to those cultured in human serum.
Anti-CD3/CD28 stimulated T cells were expanded for several days in various media adjusted with 5% human serum or 2% Physiologix. (A) When the cell exits its proliferation phase, the surface expression of CCR7 and CD45RO is measured by flow cytometry. A representative image is shown (n = 3). The cells are pre-selected for live CD8+ T cells. (B) The relative proportions of naive (CCR7+; CD45RO), central memory T cells (Tcm) (CCR7+; CD45RO+) and effector memory T cells (Tem) (CCR7; CD45RO) subsets of CD8+ T cells).
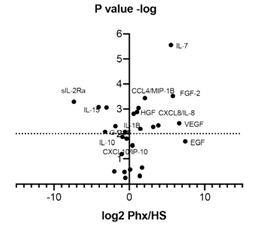
Figure S1 Relative to human serum, cytokine and growth factor richness in Phx The cytokine levels in three independent batches of Phx were collected and compared with human serum using a 31-fold array. Analyze all samples in triplicate and compare with multiple samples with an internal standard with a nine-point standard curve.
In CAR-based therapy, T cells are genetically modified by controlling antigen-specific receptors. Lentiviral vectors are commonly used as vehicles for delivering CAR genes to activated T cells. In terms of mechanism, lentivirus enters the cell and traverses the cytoplasm through endocytosis. Exogenous growth factors and cytokines are very different between Phx and HS (Figure S1), which can promote vesicle transport, mainly through the recruitment and activation of the Rab family of small GTPases [19]. The acquisition of metabolites can support the reverse transcription of lentiviral genetic material into cDNA. In order to evaluate the impact of Phx on lentiviral gene transfer,
We infected activated T cells with a lentiviral vector encoding EGFP and measured transduction after 72 hours. When the MOI was 0.5, the number of GFP-positive cells was significantly increased (2% ± 0% vs. 6% ± 0.6%, p <0.05) when the OpTmizer medium was adjusted with Phx instead of HS (Figure 3). Similar results were found at higher MOI (Figure 3). These findings provide evidence that medium supplemented with Phx can enhance transduction efficiency.
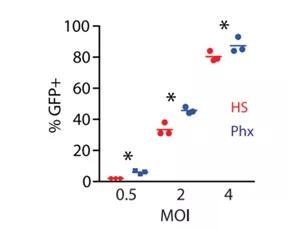
Figure 3. Phx enhances lentivirus-mediated gene expression A mixed T cell population was activated with anti-CD3/CD28 Dynabeads and cultured in OpTmizer medium conditioned by 5% human serum or 2% Physiologix. After overnight stimulation, the previously titrated GFP lentiviral surfactant was added at various dilutions. After 3 days, the efficiency of lentivirus infection was measured by flow cytometry. The average number of GFP+ cells from independent donors from 3 independent experiments ± SEM is shown. The data was analyzed by one-way analysis of variance (p <0.05). Using the Newman-Keuls multiple comparison test to compare all groups, the difference between the HS and Phx groups was statistically significant.
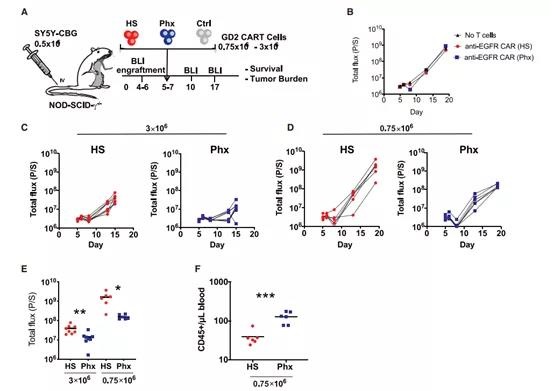
Figure 4. Study on the anti-tumor function of anti-GD2 CAR-T cells expanded in Phx-adjusted medium in vivo (A) Schematic diagram of xenograft model and anti-GD2 CAR-T cell therapy (derived from healthy donors) 0.5×106 SY5Y cells were injected iv into NSG. Activated T cells were infected with anti-GD2 CAR lentivirus and expanded in OpTmizer medium conditioned by H.S. Or Phx more than 9 days. Choose high-dose (3 106) or low-dose (0.75 106) CAR+ T cells to examine the effects of Phx and HS on the efficacy of CAR-T cells. These cells are i.v. injected into NSG mice 5 days after SY5Y injection (n = 6-8 per group). (B–H) Serial quantification of disease burden through luminescence imaging. (B) Tumor size in mice that have not received T cells or T cells expressing irrelevant CARs engineered to recognize human EGFR. Each symbol represents a rat. (C) Effectiveness of 3×106 anti-GD2 CAR-T cells expanded in Phx relative to anti-GD2 CAR-T cells expanded in HS. (D) Effectiveness of 0.75×106 CAR-T cells expanded in Phx relative to anti-GD2 CAR-T cells expanded in HS. (E) In mice treated with anti-GD2 CAR-T cells, tumor burden was quantified by bioluminescence imaging on day 10 (3×106) and day 14 (0.75×106). Values represent the mean ± SEM of each group. (F) 14 days after tumor injection, blood was collected through retro-orbital bleeding, and absolute peripheral blood CD45+ T cells were counted by TruCount assay.
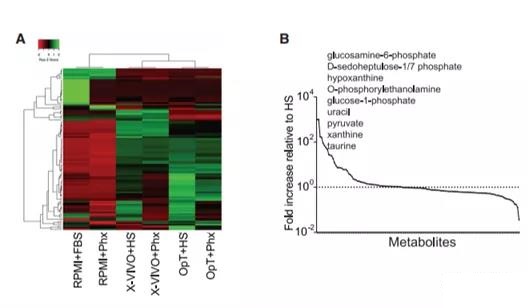
Figure 5. Metabolomics evaluation of VersusPhx-adjusted OpTmizer medium with human serum (A) The heat map illustrates the hierarchical relationship between metabolites in various media adjusted with human serum or Phx. In this grid, each row represents a unique metabolite, and each column corresponds to a unique medium formula. (B) The abundance of metabolites in Phx normalized to HS. The melanin content present in both media was normalized to the HS content. Then, the highest to lowest difference between the metabolite levels obtained by the two supplement methods is used to rearrange the metabolites on the x-axis. The dotted line represents the level in HS, and the solid line represents Phx.
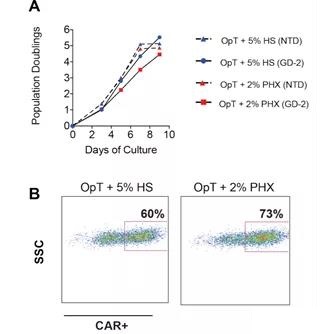
Figure S2 Expansion of anti-GD-2 CAR-T cells (A) The mixed T cells were stimulated with anti-CD3/CD28 Dynabeads and expanded in OpTmizer medium conditioned by 5% human serum or 2% Physiologix. After overnight stimulation, activated T cells were infected with GD-2 specific CAR transgene with a MOI of 4 lentivirus. At the same time, activated T cells were infected with EGFR-specific CAR transgenes of the same MOI. Enumerate the cells at the specified time point. (B) After 5 days, the CAR expression level was determined by immunostaining as described in Materials and Methods. The number of CAR+ cells is shown. The data comes from several independent experiments.
In order to evaluate the functional consequences of Phx-based CAR-T cell manufacturing, we next evaluated the anti-tumor function of CAR-T cells expanded in Phx in a neuroblastoma xenograft model. A schematic diagram of the xenograft model is shown in Figure 4A. In view of our early work in this field, we chose the E101K anti-GD2 CAR with a 4-1BB costimulatory domain. 20 GD-2 CAR-T cells were expanded in a medium containing 2% Phx versus 5% HS for 9 days (Figure S2).
Consistent with our previous findings using EGFP lentivirus, GD2 CAR expression level increased from 60% of HS to 73%. Transplant immunodeficient mice with SY5Y cells expressing Click Beetle Green Luciferase. After 5 days, the tumor cell implantation was confirmed by bioluminescence.
As shown in Figure 5A, the initial tumor burden was the same in all experimental groups. We injected 3×106 or 0.75×106 CAR-T cells intravenously (i.v.) and regularly measured the corresponding tumor size by bioluminescence, and compared the efficacy of CAR-T cells expanded in HS and Phx.
Unsurprisingly, untreated SY5Y xenografts increased exponentially over time, and control T cells expressing irrelevant CAR had the least effect on tumor cell growth (Figure 4B). As expected, high-dose (3×106 cells) CAR-T cells were expanded in HS in vitro, which significantly reduced the tumor burden.
However, GD-2 specific CAR-T cells were expanded in Phx in vitro, showing obvious superior tumor control ability (Figure 4C).
To further evaluate the effectiveness of this model, we conducted a “stress test” by reducing the infusion dose of CAR- to 0.75×106 cells. The use of this “stress test” model is increasingly recognized as an important experimental parameter that can provide insight into the potential of CAR-T cells [13,21].
In contrast, a lower dose of HS CAR-T resulted in incomplete tumor control Phx CAR-T (Figure 4D). These findings provide evidence that Phx enables CAR-T to have enhanced anti-tumor capabilities in a xenograft model (Figure 4E).
At the end of the experiment, we collected peripheral blood samples of mice treated with HS CARTS and Phx CAR-Ts. Under HS conditions, the circulating number of T cells is almost undetectable (Figure 4F). These findings are consistent with previous studies using this model [20]. The number of T cells treated with cyclic Phx increased significantly. This effect may be due to its superior replication ability and survival rate in vivo. The persistence of CAR-T cells after adoptive transfer helps to enhance tumor control, emphasizing the functional properties of Phx relative to HS. The enhanced effector function may also contribute to the increase in tumor regression observed after Phx regulation.
In order to determine the role of Phx in the effector function of CAR-T cells, we compared the killing ability of Phx and HS CARTs in a luciferase-based in vitro cytotoxicity test. After co-cultured with SY5Y target cells expressing GD-2, compared with HS CAR-T cells, Phx CAR-induced cytolytic activity was significantly enhanced within a wide range of effector: target (E:T) ratios (Figure S3 ).
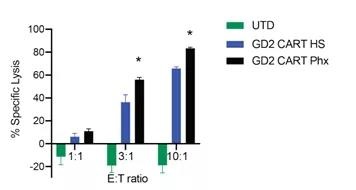
Figure S3 The in vitro cytotoxicity of anti-GD2 CAR-T cells was studied after pre-expansion in Phx
The specific cytotoxicity of anti-GD2 CAR-T cells was measured by a luciferase-based killing assay. CAR-T cells expanded in OpT+HS or OpT+2% Phx and SY5Y target cells were co-cultured in killing test medium (RPMI 1640+10% FBS) for 20 hours at the specified E:T ratio. The average value ± S.E.M. shows the value of 6 repetitions. * Px and HS at a 3: 1 E: T ratio of p <0.05; ** In the case of E: T ratio of 10:1, Phx and HS p <0.05. Using Neuman-Keuls multiple comparison post-test, the data was analyzed by two-way analysis of variance.
In view of the re-recognition of how metabolites affect T cell differentiation and effector function [22], we compared the metabolite content of Phx and HS. Using non-targeted metabolomics methods, we screened Phx and HS to reveal the unique factors of Phx, which may lead to differences in gene transduction and overall efficacy. We found that, relative to HS, several monosaccharide derivatives in Phx (including glucosamine 6-phosphate, D-heptaheptabiose 1/7 bisphosphate and glucose 1 phosphate glucose) increased by several orders of magnitude (Figure 5A) And 5B).
Compared with HS, the phospholipids and nucleotide derivatives in Phx also increased, including O-phosphoethanolamine and hypoxanthine, xanthine and uracil, respectively. Among pyruvate, pyruvate and taurine are more abundant factors that have a recognized role in the central metabolic pathway.
Our metabolomics screening determined that dipeptides containing b-alanine and L-histidine (commonly called carnosine) have a Phx that is 166% higher than HS (Figures 5A and 5B). In order to determine whether carnosine can enhance the expression of lentiviral genes in activated T cells, we added various concentrations of carnosine (3.75-30 mM) to creatine. These concentrations are similar in magnitude to other studies [23,24]. The supernatant of GFP lentivirus was added to T cells treated with sarcosine at an MOI of 0.5. We observed a dose-dependent increase in gene transduction up to 30 mM. At this concentration, carnosine effectively increased the number of GFP-expressing T cells from 31% to 43% (p = 0.05; Figure 6A). Interestingly, this effect is more pronounced in the CD4+ T cell compartment. Our data indicate that this histidine dipeptide is an important factor in enhancing parvovirus-mediated CAR transduction in activated T cells.
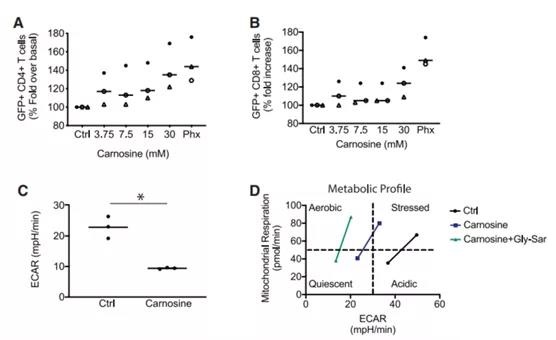
Figure 6. Carnosine enhances the expression of GFP+ lentivirus and improves the metabolic properties of T cells (A and B). Freshly isolated T cells were activated in cell culture medium containing human serum supplemented with different levels of carnosine. As a positive control, T cells were activated in a cell culture medium containing 2% Phx. After 24 hours, T cells were infected with GFP lentivirus. The ratio of live GFP+ and T cells in the CD4+ (A) and CD8+ (B) compartments is shown. As described in Materials and Methods, a bead-based flow cytometer was used to enumerate T cell subpopulations. The value is expressed as a percentage of OpT+HS. The mean±SEM of independent donors from 3 independent experiments is shown. (C) Activation of T cells with Dynabeads. After overnight stimulation, the cells were switched to bicarbonate-free XF analysis medium containing 30 mM carnosine. The metabolic parameters are measured by the extracellular flux assay. Shows the steady-state extracellular acidification rate (ECAR). Values are the mean ± SEM of independent donors from three independent experiments. *p<0.01, comparison between control and carnosine, unpaired Student’s t-test analysis. (D) Use Dynabeads to activate T cells for 1-3 days. Switch the cells to bicarbonate-free XF analysis medium containing 10 mM carnosine and 30 mM glycylsarcosine (Gly-Sar).
The strategy of limiting glycolytic metabolism during in vitro culture has resulted in T cells with enhanced anti-tumor function [25-27]. The basic premise of these studies is that glycolysis is the effect of people’s poor understanding of the mechanism that differentiates T cells. Subpopulations with low implantation and durability after adoptive transplantation. The decomposition of ethylene glycol eventually forms lactic acid (H+ La), which is decomposed into an oxidizable fuel source, lactic acid and corresponding protons. Both La and H+ are exported outside the cell. Glycolysis-induced acidification has harmful effects on T cell function and quality, and acidification has intracellular and extracellular effects.
To test the ability of carnosine to neutralize extracellular acidification, we performed an extracellular flux (Seahorse) assay. After stimulation with Dynabeads overnight, the T cells were switched to Seahorse analysis medium containing HEPES and supplemented with 30 mM Carnotine. Before use, adjust the pH of the medium to pH 7.4. As shown in Figure 6C, carnosine effectively buffered the accumulation of extracellular protons. The steady-state extracellular acidification was significantly reduced from 23 mpH/min (p <0.05). Carnosine can also change the metabolic mode of activated T cells from glycolysis to oxidation (Figure 6D).
In the process of cell culture, the oxidative metabolism status is better than the glycolytic metabolism status, because it reaches the highest level in the progeny of T cells with excellent anti-tumor function. 25-27 In order to distinguish the intracellular and extracellular effects of carnosine, we added Gly-Sar to competitively inhibit the uptake of carnosine through the cell surface transporter PEPT1. Gly-Sar enhances the ability of CAR neuraminic acid to limit extracellular acidification and enhances the effect of sarcosine on the overall metabolic phenotype (Figure 6D).
Summary
These findings highlight the therapeutic potential of Phx as an alternative to HS. Produces enhanced dehydrogenation and efficacy of CAR genes in cancer xenograft models. Our findings also highlight the therapeutic value of identifying and including biogenic amines (such as carnosine). Compared with HS, carnosine is moderately enriched in Phx to improve the overall quality of the cell culture medium for CAR-T cell therapy.
Discussion
We show that CAR-T cells grown in Phx exhibit enhanced potency relative to HS. Anti-GD2 CAR-T cells expanded in Phx are functionally superior to CAR-T cells expanded in HS. When comparing a limited number of injected CAR-Ts, an effect can be produced. The increase in the accumulation of Phx-treated T cells in the peripheral blood is consistent with the tumor clearance rate, which may be the result of increased replication capacity, cytolytic activity and survival rate. The difference in CAR-T cell activation is unlikely to lead to its excellent therapeutic effect, because the total antigen density of all groups is the same before CAR-T is injected.
Our data encourages further optimization of media formulations used in adoptive immunotherapy. We show that Phx enhances the efficacy of CAR-T cells redirected to solid tumor GD-2 antigen. Expanding CAR-T cells in the medium under the condition of growth factor extracts can enhance their persistence and effector functions after transplantation. Since most cells are excluded from the serum used for cell culture, our findings raise important questions in the design of the medium. Are the current in vitro medium preparations suboptimal for CAR-T cell function? Are CAR-T cells hindered by our ex vivo expansion conditions? Our data can accelerate future progress by including systemic factors that allow for enhanced implantation after reinfusion.
We tested Phx’s ability as a serum-free alternative in a wide range of media. We have proved that Phx is a conditioner suitable for research grade RPMI medium and clinical grade X-Vivo15 and OpTmizer medium. The medium supplemented with Phx supports the ex vivo expansion of T cells isolated from healthy volunteers. In RPMI supplemented with 2% Phx and 10% FBS, a moderate reduction in the proliferation ability of T cells was observed. In these cases, the stoichiometric imbalance of biologically active peptides, growth factors, and lipids may be the basis for this effect.
Activated T cells are genetically modified by CAR to confer antigen specificity, direct signal transduction, promote effector functions and reprogram metabolism. The delivery of CAR genes has been optimized through the use of HIV-based lentiviral plasmids, but it is still expensive in the clinical field. In cells activated in Phx-conditioned medium, lentivirus-mediated gene delivery was significantly enhanced by 2 to 3 times. Our findings suggest the role of carnosine, during which carnosine is moderately elevated in Phx relative to HS. Phx contains a large number of other factors that have not been tested, and may also support this process.
Effector: The target ratio reflects the dynamic balance between the two. The number of functionally competent T cells and their corresponding targets. Many internal factors of T cells (including replication ability, differentiation and survival) combine to change the balance, which facilitates the cytolytic activity of T cells to exceed the proliferation of tumor cells. When the infusion dose was 0.75e6, only the CAR-T amplified in Phx exceeded the critical set point necessary to control the tumor burden. These findings indicate that the medium formulation is an important parameter that affects the effectiveness of CAR-T cells.
In summary, our work provides evidence that the presence of external factors in the culture medium during the manufacturing process can further improve the effectiveness of CAR-T cells. These findings shed light on the mechanisms that regulate gene transfer, metabolic adaptability, effector function and persistence. The process essential for CAR-T in vivo behavior may gradually improve the production of T cells in vitro for therapeutic purposes.
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



