Autoimmune and Inflammatory Diseases: 30 years experience and reflection
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
Autoimmune and Inflammatory Diseases: 30 years experience and reflection
Autoimmune and Inflammatory Diseases: 30 years experience and reflection. The deciphering of the human genome coupled with advances in biotechnology, especially the milestone discovery of monoclonal antibodies (mAbs), has enabled biological therapy to greatly improve the survival time and survival status of patients.
Currently, more than 300 biological therapies have been approved by the U.S. Food and Drug Administration (FDA), many of which are approved for the treatment of immune and inflammatory diseases.
While these new drugs have brought huge benefits to mankind, we have also learned a lot, and have reflected on the experience of developing these drugs.
1. Choose a clinical endpoint that fits the biological pathway
There may be heterogeneity in different clinical manifestations of patients.
In psoriasis and related psoriatic arthritis (PsA), targeting the IL-23/17 pathway can significantly improve skin performance. These drugs prove clinical improvement of arthritis, but the benefit is lower than that observed in the skin, which means that other inflammatory pathways are also involved in affecting arthritis.
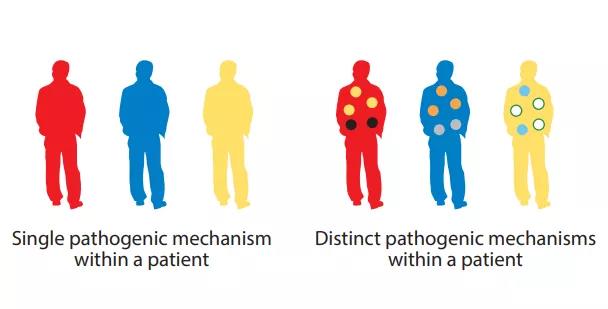
Similarly, IL-5 targeted therapy can significantly relieve asthma exacerbations, but has a relatively small effect on improving baseline lung function and symptoms. Therefore, different pathophysiological mechanisms can drive different clinical manifestations, and choosing the appropriate clinical endpoint is crucial. This intra-disease heterogeneity further indicates that in some diseases, combined treatments may be required to maximize the clinical benefit of specific patients.
Biomarkers are critical to the success of drug development
Whether a drug is effective depends on whether it can achieve pre-specified clinical endpoints in key trials. In order to increase the likelihood of success, biomarkers help maximize clinical benefit and the likelihood of success in the drug development process.
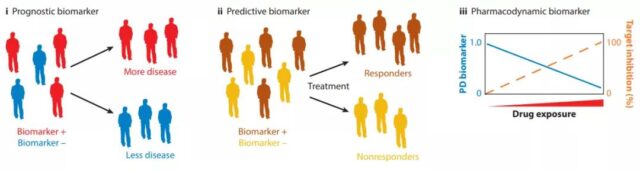
The subgroups of patients identified by prognostic biomarkers have different clinical processes. Predictive biomarkers can be used to identify patients who are more likely to benefit from treatment before treatment. Pharmacodynamic biomarkers report the ability of the drug to bind to the target. , Provides important information about drug dosage and timing.
The use of predictive, prognostic, and pharmacodynamic biomarkers in drug development can alleviate the problem of patient heterogeneity, enrich the measurement methods of clinical outcomes, and determine whether sufficient targeted inhibition has been achieved.
2. Experience of failed clinical trials
A negative clinical trial result does not necessarily mean that the biological pathway involved has nothing to do with the disease. There are at least four main reasons for clinical trial failure:
2.1 Drug design:
Insufficient research on the molecular properties of candidate drugs, including target specificity, affinity and potency, non-targeting effects that cause adverse events of restricted targeting inhibition, poor biodistribution of the drug to the disease site (such as the brain), and pharmacokinetics Poor kinetics may lead to negative results. The rigorous application of sensitive pharmacodynamic biomarkers can usually identify these causes.
2.2 Research design:
Failure to develop appropriate entry criteria for the study population with sufficient disease activity or the possibility of disease progression during the study period may have a negative impact on the sensitivity of clinical trials to detect treatment effects. The placebo group needs to receive concomitant standard treatment (SOC) and maintain high compliance. If the clinical effect of the placebo group is not explained, the treatment window for measuring the effect will also be reduced. Finally, matching appropriate clinical endpoints with targeted biological pathways is essential for multifactorial diseases with heterogeneous clinical manifestations.
2.3 Patient selection:
If the proper distinction is not made, disease heterogeneity will dilute the therapeutic effect of clinical trials. Therefore, it is important to use appropriate biomarkers as diagnostic criteria. In the second stage of the proof-of-concept test, it is necessary to ensure a sufficient number of patients (including positive and negative diagnosed patients) in order to provide the appropriate ability to detect the subgroup of patients with positive diagnosis The clinical benefits of the test to verify the diagnosis. In many cases, a suitable boundary needs to be provided to define a positive diagnosis.
2.4 Target selection:
A well-designed experiment should be able to rule out causes 1-3. If even this is the case, negative results are still obtained, which may mean that although preclinical information leads to the target of the indication, the treatment concept is actually still wrong.
3. Treatment targets and drug development
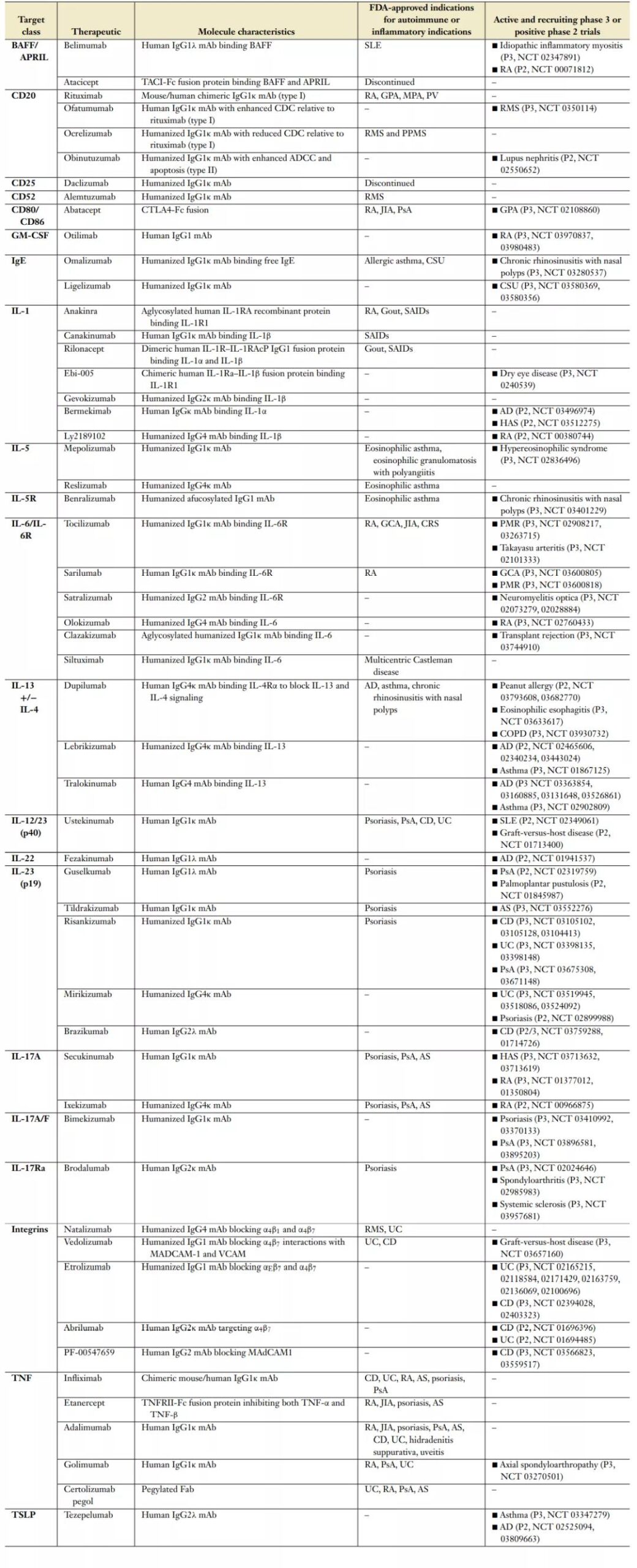
The following table lists the biotherapies approved by the FDA for autoimmune disease indications and in key phase 3 clinical trials or positive phase 2 clinical trials (data update date: April 2020).
Autoimmune and Inflammatory Diseases: 30 years experience and reflection
Interleukin-1
IL-1α and IL-1β are positioned to respond quickly to the inflammatory response mediated by microorganisms under sterile conditions. When epithelial cells, endothelial cells and platelets respond to tissue damage, IL-1α acts as an alarm.
In contrast, the myeloid-derived subtype IL-1β is synthesized as an inactive protein and requires proteolytic processes driven by inflammasomes to be active. IL-1α/β belongs to the larger IL-1 cytokine family, which uses IL-1 receptor co-protein (IL-1RAcP) to pair with IL-1R1 subunits to generate MyD88-dependent signals. IL-1α/β function is also regulated by natural antagonists IL-1Ra, sIL-1RAcp and decoy receptor IL-1R2. The dysregulation of IL-1α/β expression is related to a variety of systemic autoinflammatory diseases (SAID) characterized by fever, rash, arthritis, and organ-specific inflammation.
The FDA has approved three different types of biological therapies, but they all target IL-1.
- Anakinra is an improved version of the natural antagonist IL-1Ra, which inhibits both IL-1α and IL-1β. Anakinra is approved for the treatment of RA and CAPS, with a short half-life of 5-6 hours, so it needs to be injected subcutaneously every day. Anakinra is also effective against gout. Monosodium urate crystals stimulate NLRP3 activation and IL-1β production.
- Canakinumab is an IL-1β neutralizing antibody that is administered subcutaneously every 4-8 weeks and is approved for the treatment of systemic juvenile idiopathic arthritis (SJIA) and SAIDs.
- Rilonacept encodes the extracellular domains of IL-1R and IL-1AcP, and is connected to the Fc part of IgG to preferentially neutralize IL-1β. It is approved for weekly subcutaneous administration of SAIDs.
Interleukin-6
IL-6 is a member of the gp130 cytokine family and is a central component of many homeostatic and inflammatory processes.
IL-6 was originally identified as a T cell-derived factor that promotes B cell differentiation, and it is now being valued for its pleiotropic activity in adaptive immunity. It helps the differentiation of Th17 and T follicular helper cells (Tfh), drives the differentiation of myeloid cells, and works with Th2 cytokines to promote the polarization of macrophages into a pro-fibrotic phenotype. IL-6 plays an important role in the acute phase reaction of the liver. It increases inflammatory proteins including C-reactive protein (CRP), enhances the combination of complement and pathogens or dead cells, and also has an important impact on endothelial cell function and epithelial cell integrity.
The extensive biological activity of IL-6 stems from its complex nature of regulating multiple cellular targets. IL-6 is synthesized by a variety of cells, and activates target cells through three different cell surface signaling mechanisms (ie, classical signaling, trans signaling, and trans presentation), and finally generates JAK/STAT signaling.
Tocilizumab and sarilumab are two IL-6R blocking monoclonal antibodies approved by the FDA, and many other drugs are in clinical development. Tocilizumab and sarilumab are approved by the FDA for the treatment of moderate to severe RA, where IL-6 levels in the synovial fluid of affected joints are elevated, and serum IL-6 levels are related to disease activity. Both of these drugs can improve inflammatory symptoms and reduce imaging progression in RA patients.
Cytokine release syndrome (CRS) is an acute systemic inflammatory disease associated with many antibody-based therapies, chemotherapy and immunotherapies involving T cells (such as CAR-T cells), and severe infections. CRS, which is related to the participation of T cells in therapy, is believed to be the production of TNF-α by activated T cells, which in turn triggers the production of IL-6 and IL-1β by monocytes and activated macrophages. Relief of CRS symptoms (such as fever and hypotension) is usually achieved after a single dose of Tocilizumab. Administration of tocilizumab does not seem to affect the anti-tumor effect of T cells involved in the therapy.
Tumor necrosis factor
TNF-α is mainly produced by immune cells and endothelial cells, and is significantly up-regulated by pro-inflammatory signals and bacterial products; TNF-β (LTα) is mainly produced by lymphocytes.
TNF-α is expressed as a trimeric membrane-bound form (mTNF) and undergoes proteolytic cleavage to produce soluble trimer sTNF. TNFR1 is widely expressed, while TNFR2 is mainly expressed in neurons, immune cells and endothelial cells. TNF-α has a pleiotropic function. TNF-α is necessary for the best defense against pathogens, the normal development of lymphoid organs, and the important repair effects in neuronal remyelination, heart remodeling, and cartilage regeneration.
There are currently five TNF inhibitors approved by the FDA for clinical use, all of which target TNF-α, of which etanercept additionally inhibits TNF-β. The molecular types of tumor necrosis factor antagonists reflect the development of biotherapeutics in the past two decades. Etanercept (TNFR2-Fc) is the first Fc fusion protein approved by the FDA; infliximab is the first-generation chimeric monoclonal antibody; adalimumab is a human monoclonal antibody derived from phage display; golimumab is derived from expressing human IgG Human monoclonal antibody for transgenic mice; certolizumab-pegol is a humanized Fab fragment isolated from mouse hybridomas, which can prolong its half-life in vivo by pegylation.
Most tumor necrosis factor antagonists show common clinical efficacy.
However, etanercept was ineffective in a randomized double-blind placebo-controlled trial of Crohn’s disease (CD), which may reflect differences in the treatment mechanism of TNF inhibitors. In addition to neutralizing sTNF-α, anti-TNF antibodies and certolizumab-pegol (rather than etanercept) can induce lamina propria T cell apoptosis by binding to mTNF or blocking anti-apoptotic signals. In addition, anti-TNF monoclonal antibodies (but not etanercept or certolizumab-pegol) can induce M2 type wound healing macrophage responses through an Fc-dependent mechanism.
Therefore, the mechanism difference between TNF antagonists may be the reason for their different clinical effects in CD.
CD20
CD20 is a B cell marker whose expression starts from early pre-B cells, but is lost during the terminal differentiation into plasma cells. It is a tetrapeptide protein, which is necessary for the optimal function and immune response of B cells.
Rituximab is a chimeric monoclonal antibody and the first anti-CD20 monoclonal antibody approved by the FDA for the treatment of B-cell non-Hodgkin’s lymphoma. Based on its surprisingly good safety in oncology, researchers began to explore its application in patients with severe autoimmune diseases. Rituximab is currently approved by the FDA for the treatment of various autoimmune diseases. Since CD20 is neither expressed on HSC nor terminally differentiated plasma cells, the selective targeting of CD20+ B cells by anti-CD20 monoclonal antibodies has different immunological consequences from congenital B cell deficiency. For example, after treatment with anti-CD20 antibodies, serum IgG levels have no significant effect, while severe X-linked angammaglobulinemia (in which B cells are underdeveloped) is observed to lack immunoglobulin.
A recent phase 2 study used obinutuzumab, a type II anti-CD20 monoclonal antibody with the ability to enhance ADCC and induce B cell apoptosis. The clinical benefit of patients with lupus nephritis has been reported (NCT 02550652). Further research on obinutuzumab is needed to confirm whether additional B cell depletion is beneficial for lupus nephritis.
BAFF/APRIL tumor necrosis factor superfamily member
BAFF and APRIL are two type II transmembrane proteins of the TNF ligand superfamily. Their receptors BAFFR, BCMA and TACI play an important role in the survival, maturation and function of B-lineage cells.
Serum BAFF and APRIL levels are elevated in many autoimmune diseases, including SLE, Sjögren syndrome, and RA.
Belimumab is a human IgG1λ monoclonal antibody, approved for the treatment of active autoantibody-positive SLE. It inhibits sBAFF trimer, but does not bind to mBAFF or APRIL. In key clinical trials, belimumab showed greater improvements in SLE response index and health-related quality of life endpoints compared with the SOC group. In addition, belimumab reduces the number of primitive B cells, activated B cells and plasma cells in the circulation; anti-dsDNA levels are reduced and complement levels are normalized. Consistent with the lack of effects on circulating memory B and T cells, after one year of treatment, the pre-existing antibodies against pneumonia, tetanus and influenza A were not affected.
Atacicept is an Fc fusion protein fused to the extracellular domain of TACI, which binds BAFF, APRIL and BAFF/APRIL heteromers. After applying Atacicept treatment in patients with systemic lupus erythematosus, serum IgM (∼70%), IgG (∼30-40%) and IgA (∼50-60%) dropped rapidly, and anti-double-stranded DNA antibodies dropped by ∼40% . After the treatment was terminated, the antibody and autoantibody levels returned to the pretreatment level.
Integrin family
Integrins regulate the transport of immune cells by regulating the adhesion of white blood cells to blood vessels and promote the extravasation of white blood cells into tissues. The integrin heterodimer is composed of the pairing between 18 α subunits and 8 β subunits, forming 24 different receptor complexes, which have differences in expression patterns, ligand specificities and functions.
In MS, α4β1 (CD49d/CD29, VLA4) is expressed on T and B cells and promotes the extravasation of lymphocytes into the central nervous system (CNS) through the combination of VCAM1 and blood-brain barrier endothelial cells. In the gastrointestinal tract, α4β7 expressed on T cells can bind to the MADCAM-1 in the high endothelial venules of Peyer’s plaque and the lamina propria venules to promote the extravasation of pathogenic T cells in inflammatory bowel disease (IBD).
Natalizumab is an α4-blocking monoclonal antibody, approved for the treatment of patients with CD and RMS. However, the mechanistic basis for the effectiveness of RMS in inhibiting lymphocytes from entering the central nervous system also provides a basis for the development of rare cases of progressive multifocal leukoencephalopathy (PML).
The second-generation antibodies, including vedolizumab (selective for α4β7 complexes) and etrolizumab (anti-β7), reduce the possibility of PML through intestinal-specific integrins for IBD. Vedolizumab can induce clinical response and remission, and the FDA has approved it for ulcerative colitis (UC) and CD. In addition to blocking the α4β7:MADCAM1 interaction, etrolizumab also interferes with the retention of αEβ7+IELs by E-cadherin + intestinal epithelial cells.
In a phase 2 clinical trial, compared with the control group, the use of etrolizumab for UC patients is more likely to lead to clinical remission. Since αEβ7+IELs are important contributors to inflammatory cytokines in UC patients, blocking α4β7 and αEβ7 integrins may have additional clinical benefits.
The anti-αL antibody Efalizumab blocks the interaction of αLβ2 (LFA1) expressed by T cells and B cells with ICAM1. In addition to blocking integrins, Efalizumab also down-regulates the expression of LFA-1, which is necessary for T cells to form synapses with antigen presenting cells (APC). Efalizumab is approved by the FDA for the treatment of moderate to severe plaque psoriasis. However, 4 patients treated with Efalizumab for more than 3 years developed PML, and the drug has been withdrawn from the market.
Interleukin-12 and -23 and their downstream effectors
IL-12 and IL-23 are two closely related APC-derived heterodimeric cytokines, composed of p35 and p19 subunits, respectively, which pair with a common p40 subunit. For these two cytokines, the main response cells are T cells and innate lymphocytes (ILCs). In different cell types, IL-12 up-regulates the expression of Th1 main transcription factor T-bet and induces the production of IFN-γ.
In a similar manner, IL-23 stimulation induces the expression of transcription factor RORγt and the expression of downstream cytokines IL-17A, IL-17F, IL-22 and GM-CSF. Preclinical models of the disease and genome-wide association studies indicate that the IL-12/23 pathway is involved in inflammatory bowel disease and psoriasis.
Various therapies designed to interfere with various components of the IL-12/23 pathway have entered the clinic. Two monoclonal antibodies (ustekinumab and briakinumab) target the p40 subunit, thereby neutralizing IL-12 and IL-23. Recently, the IL-17 family of effector cytokines has attracted widespread attention. There are five monoclonal antibodies that selectively neutralize IL-17A; two monoclonal antibodies neutralize IL-17A and F; brodalumab targets IL-17RA, one IL -17 Co-receptor for members of A, B, C, E, and F. However, although the different functions of IL-17B-E have been described, these cytokines are not regulated by IL-23, and their role in human diseases has not been fully studied.
In addition, studies on blocking IL-22 (fezakinumab), IFN-γ (fontolizumab) and GM-CSF (namilumab and mavrilimumab) treatments will provide further insights, although they are still in early research.
Opportunities and Challenges (Autoimmune and Inflammatory Diseases: 30 years experience and reflection)
In the past thirty years, advances in biological therapy have brought tremendous benefits to patients, and have gained an important understanding of basic immunology and disease pathogenesis.
These clinical trials also revealed major challenges in the development of new therapies, partly due to disease heterogeneity and clinical research design, including patient selection, endpoint selection, and biomarker discovery and research.
There are still a large number of unmet clinical needs in the research and development of new drugs, and tools and methods for discovering new targets and new biomarkers continue to emerge, some of which are exciting developments. Many of the challenges of understanding the biology of human diseases and developing new therapies are balanced by these emerging new technologies and insights. Their application will undoubtedly enhance our understanding of human diseases and enhance our ability to provide clinical benefits for unmet medical needs.
references: (Autoimmune and Inflammatory Diseases: 30 years experience and reflection)
1.30 Years of Biotherapeutics Development-What Have We Learned? Annu Rev Immunol. 2020 Apr 26;38:249-287
— THE END —
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



