Controversial AZ drug: Over one-third of patients have cerebral edema
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
Controversial AZ drug: Over one-third of patients have cerebral edema
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
In the controversial new drug for Alzheimer’s disease, more than one-third of patients have cerebral edema after treatment.
June 7, 2021, the FDA announced the accelerated approval Biogen of monoclonal antibody drugs Aducanumab (trade name Aduhelm) on the market for the treatment of Alzheimer’s disease -derived mild cognitive impairment (MCI) and mild Alzheimer’s Disease .
This is the first new drug approved by the FDA for the treatment of Alzheimer’s disease since 2003, and it is also the first drug that can prevent the progression of the disease.
 aducanumab
aducanumab
After Aducanumab was approved by the FDA, it immediately caused a huge controversy. Clinical trials showed that this monoclonal antibody drug can clear β-amyloid (Aβ) in the brain , but there is insufficient evidence that it can slow or prevent Alzheimer’s disease. the progression of the disease , three FDA reviewers resigned in protest.
The drug is expensive, up to 56,000 US dollars per year . Due to the lack of evidence of effectiveness and the risk of side effects, although the drug was approved by the US FDA for marketing, major insurance companies in the United States refused to pay for the treatment of the drug.
On November 22, 2021, JAMA Neurology published a paper titled: Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease .

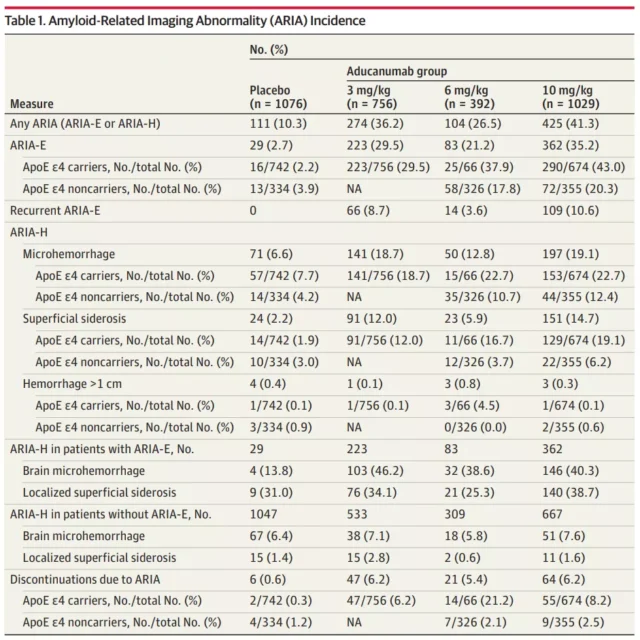
The paper reports on two large phase 3 clinical trials of aducanumab in the treatment of Alzheimer’s disease, and focuses on the amyloid-related imaging abnormalities (ARIA) in patients during treatment .
The results showed that in 1029 patients 10mg / kg dose group, 425 patients (41.3%) experienced an amyloid-related imaging abnormalities (ARIA) problems , 362 patients (35.2%) occurred ARIA brain edema , wherein 94 People have symptoms such as headache, confusion, dizziness and nausea.
197 patients (19.1%) had ARIA microbleeds, and 151 patients (14.7%) had ARIA surface iron deposits. Among these patients with side effects, 14 were in serious condition.
These two large-scale Phase 3 clinical trials showed that patients receiving more than 40% of aducanumab treatment appeared after amyloid-related imaging abnormalities (ARIA) problem , which has about a quarter of the emergence of symptoms.
Tortuous road
In November 2007, Biogen from Neurimmune the introduction of monoclonal antibody drug candidates for the treatment of early stage Alzheimer’s disease company aducanumab , the monoclonal antibody drugs can selectively brains of patients with Alzheimer’s disease in β- starch Aβ -like protein (Aβ) deposits and binds, and then activates the immune system to clear the Aβ deposit protein in the brain.
Since October 2017, Bojian and Eisai have cooperated on the development and commercialization of this monoclonal antibody globally.
However, March 21, 2019, Biogen announced that clinical trials ENGAGE and EMERGE Phase III global termination aducanumab of two code-named, independent commission to assess its likely be difficult to achieve the desired effect. As soon as the news came out, Bojian’s stock price plummeted 27%, and its market value shrank by US$15 billion.
Enjoying the cool shade, October 22, 2019, Biogen announced that its medical statistics division after analyzing larger data sets, found aducanumab compared to placebo had a marked effect. So re-submitted the “Biologics License Application” (BLA) application to the FDA . Affected by this news, Bojian’s share price soared 40%.
However, this is not over yet.
On November 6, 2020, the FDA’s Peripheral and Central Nervous System Drug Advisory Committee re-applied for Bojian , and most members voted against it.
April 2021, three members of the FDA Peripheral and Central Nervous System Drugs Advisory Committee –Caleb Alexander, Scott Emerson and Aaron Kesselheim in JAMA published journal article criticized aducanumab , says Biogen This is the first archery, after painting the target.
Experienced a life-giving aducanumab this point is still uncertain, the outside world for whether the FDA will approve the Bohai health and Eisai Alzheimer’s drug candidates developed aducanumab still full of doubt, but also make them drug development story in 2021 the most talked about ( One) .
The first new drug for Alzheimer’s disease approved by the FDA in 18 years
And in the US local time on June 7, the FDA announced the accelerated approval Biogen developed aducanumab treatment of Alzheimer’s disease patients. Aducanumab has also become the first new treatment approved for Alzheimer’s disease since 2003.

Reference:
https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
https://www.nejm.org/doi/full/10.1056/NEJMoa2100708?query=featured_home
https://www.nature.com/articles/d41586-021-03410-9
https://jamanetwork.com/journals/jamaneurology/fullarticle/2786606
Controversial AZ drug: Over one-third of patients have cerebral edema
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



