Antibody Drugs: The Developmental Stages of Therapeutic Antibodies
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
Antibody Drugs: The Developmental Stages of Therapeutic Antibodies
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Antibody Drugs: The Developmental Stages of Therapeutic Antibodies.
A major breakthrough in antibody technology was the birth of monoclonal antibody technology.
In order to facilitate the distinction, antibodies prepared by immunizing animals with antigens to obtain antiserum are called polyclonal antibodies.
In 1975, British scientist Milstein and French scientist Kohler fused antibody-producing B lymphocytes with tumor cells to form hybridoma cells.
Hybridoma cells have the characteristics of parental cells, which can not only produce antibodies, but also have the characteristics of infinite proliferation of tumor cells, so as to continuously secrete monoclonal antibodies.
Monoclonal antibodies have many advantages and are of epoch-making significance in the clinical application of antibodies.
The two scientists were therefore awarded the 1984 Nobel Prize in Physiology or Medicine.
Since the first therapeutic antibody entered the clinic in 1986, therapeutic antibodies have developed rapidly.
So far, nearly one hundred therapeutic antibody drugs have been approved by the FDA, which have become an important part of modern biomedicine.
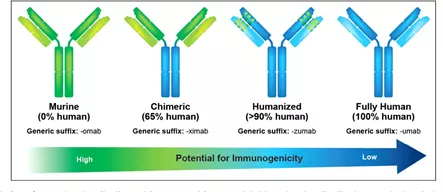
With the development of modern technology, therapeutic antibodies have gone through different development stages such as murine antibodies, chimeric antibodies, modified antibodies and resurfaced antibodies (partially humanized antibodies), as well as fully humanized antibodies.
The first generation: mouse monoclonal antibody (momab)
In 1986, the year after Milstein and Kohler won the Nobel Prize for their monoclonal antibody hybridoma technology, Johnson & Johnson’s Orthoclone OKT3 became the first FDA-approved monoclonal antibody to prevent the host after kidney transplantation exclusion.
But it wasn’t until nine years later that a second antibody drug, ReoPro from Eli Lilly and Johnson & Johnson, was launched in the U.S. in 1995 and used to inhibit blood clots.
The first monoclonal antibody drug, Orthoclone OKT3, came from mice, and its amino acid sequence is of mouse origin.
Mouse-derived antibodies often encounter some problems when they are administered to patients:
1) The human body regards these monoclonal antibodies as foreign proteins, which will cause immune rejection.
2) Immune rejection allows the monoclonal antibodies to be quickly removed from the patient’s body, greatly reducing their expected efficacy. Especially in the case of chronic diseases requiring long-term use, mouse-derived monoclonal antibodies have little effect in subsequent injections;
3) In a few cases, mouse-derived antibodies can cause severe allergic reactions and even lead to the death of individual patients.
Therefore, the sales of early monoclonal antibodies have not taken off – the annual sales of Orthoclone OKT3 are only about 10 million US dollars.
In order for monoclonal antibodies to gain a foothold in the arena and have wider medical applications, they must be transformed into humanized antibodies or humanized antibodies.
Second generation: human-mouse chimeric monoclonal antibody (ximab) and humanized monoclonal antibody (zumab)
Here we need to distinguish between humanized antibodies and human antibodies. Humanized antibodies are generally based on murine antibodies.
By replacing protein fragments and replacing part of the amino acid sequence, the final amino acid sequence of the antibody is closer to human.
A human antibody is any antibody that can be expressed by human B cells, and its amino acid sequence is 100% encoded by human genes.
In the 1990s, dozens of biotech companies emerged, mainly in the United States.
All of them have unique skills – their technology platforms are built around how to humanize antibodies or directly generate human antibodies, and their purpose is to carry forward antibody drugs.
Companies focusing on the research and development of antibody drugs were divided into two major schools in the rivers and lakes at that time.
The first school can be called antibody protein engineering school or humanization school.
After the monoclonal antibody is produced in mice, part of its amino acid sequence is either replaced or assembled by splicing, and its ultimate goal is to neither cause immune rejection in humans nor reduce its affinity for the target antigen.
This genre is further divided into two levels. The first level is the chimeric antibody: the constant region of the antibody is replaced with the human amino acid sequence.
About 33% of the amino acid sequence of the chimeric mAb protein is derived from mouse, and the remaining 67% is of human origin.
The second level is humanized antibody, that is, after obtaining a mouse antibody against a certain antigen, only a few regions (CDR regions) that recognize the antigen are taken and transplanted into human antibodies.
Humanized sequences account for 90% of the humanized mAbs. Humanized mAbs clearly have advantages over chimeric mAbs, with a lower risk of immune rejection or hypersensitivity. The representative company of humanized mAb technology is Protein Design Labs, or PDL.
The humanization of several of Genentech’s well-known monoclonal antibodies—Herceptin, Xolair and Avastin—requires technology licenses from PDL.
The biggest disadvantage of humanized mAb technology is the lack of a general approach.
Humanization of each antibody molecule requires case-by-case analysis, molecular modeling, extensive engineering, and trial and error.
Even so, humanized mAbs cannot completely avoid the risk of immune rejection or hypersensitivity due to the presence of murine sequences.
 The third generation: fully humanized monoclonal antibody (mumab)
The third generation: fully humanized monoclonal antibody (mumab)
The second genre of antibody drugs is fully human monoclonal antibodies. This genre is divided into two major schools: phage display and transgenic mice.
Antibody production using phage display technology completely avoids the use of animals.
In this technology, a gene bank expressing the variable regions of numerous human antibodies is first established by PCR technology using phage plasmids as vectors.
When E. coli was transfected with these plasmids, millions of phages were released. Each phage displays a unique antibody variable region segment on the surface.
After the solution containing these phage mixtures has flowed through the solid substrate attached to the specific antigen, it is often the phage particles presenting the specific antibody that stick to the surface of the substrate and cannot be washed away.
The gene of the specific antibody is further amplified and purified. Representative companies of this genre include CAT and Dyax.
Although transgenic mouse technology debuted late, its technical advantages are the most obvious.
This technology destroys the mouse’s own antibody expression system by means of transgenic, and then introduces the human antibody production system.
This transgenic mouse can directly produce fully human antibodies against a certain antigen.
Phage display and transgenic mice each have their own merits in execution. Generally speaking, phage display technology is “fast first and then slow”, that is, it is very fast to find an antibody against a certain target protein, but the affinity of the selected antibody to the target protein is often not high, so manual fine-tuning is required to replace individual amino acid.
This step of optimization is time-consuming and labor-intensive, and even if the optimized antibody is compared with the antibody produced by the transgenic mouse, the affinity may differ by an order of magnitude.
In addition, some amino acids need to be replaced during the optimization process, which introduces the risk of immune rejection. Transgenic mouse technology is “slow first, then fast”, and it takes several months for the first steps to inject antigen into mice, generate specific antibodies, and prepare hybridoma cells.
But once the initial antibodies are produced, the optimization process continues in mice, fast and well, without the fear of immune rejection.
Here I have to mention the world’s first fully human antibody-Adalimumab (Hummerole).
Adalimumab began in 1993 as a joint research effort between BASF’s subsidiary BASF Knoll and Cambridge Antibody Technology (CAT).
Cambridge Antibody Technology used TNFα as antigen and obtained fully human antibody D2E7 in in vitro screening using their unique phage display technology.
In the subsequent research, BASF Knoll further improved the fully human antibody D2E7, and completed the preliminary production process development and clinical application.
In June 2002, Abbott Pharmaceuticals (Abbott) acquired BASF Knoll for US$6.9 billion, obtained the development, production and sales rights of the fully human antibody D2E7, and finally brought adalimumab to the market.
Since it was first approved by the FDA in 2002, adalimumab has accumulated sales revenue of US$116.1 billion.
In 2017, the growth rate of the US market was 18.5% and the global market growth rate was 14.6%. The double-digit increase set an annual sales record for a single drug.
Antibody Drugs: The Developmental Stages of Therapeutic Antibodies
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.