Clinical results of Moderna’s Omicron vaccine are more broad-spectrum than original vaccine
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
- Statins Lower Blood Lipids: How Long is a Course?
- Warning: Smartwatch Blood Sugar Measurement Deemed Dangerous
Clinical results of Moderna’s Omicron vaccine are more broad-spectrum than original vaccine
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
Clinical results of Moderna’s Omicron vaccine are more broad-spectrum than original vaccine
The current popular new coronavirus mutant strains are all branches of Omicron.
Compared with the original virus, the Omicron mutant strain has many mutations on the key antigenic spike protein.
Several studies have shown that today’s Omicron variant is more transmissible and has significantly higher immune evasion than the original strain.
It is worth mentioning that the mutant strain of Omicron showed strong immune escape to the vaccine designed with the original virus strain as the blueprint.
This also means that vaccination with existing vaccines may not be effective in preventing and controlling the mutant strain of Omicron. Because of this, many domestic and foreign companies have begun to invest in vaccine research and development for Omicron.

Moderna’s improved vaccine trial results announced, Safe and effective against Omicron
Recently, a number of pharmaceutical companies, including Pfizer/BioNTech and Moderna, have successively carried out R&D and clinical trials of the Omicron vaccine.
On June 8, Moderna announced the first clinical trial results of a new-generation COVID-19 vaccine containing the Omicron vaccine.
This vaccine is a combination of Moderna’s original vaccine mRNA-1273 and a candidate vaccine against Omicron, an improved version of the mRNA COVID-19 vaccine.
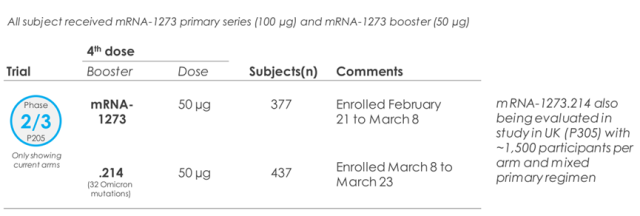
Figure 1 Moderna’s improved version of Omicron vaccine (Source: [1])
In this phase II/III clinical trial, subjects were vaccinated with three doses of Moderna’s original vaccine mRNA-1273, with the first two doses being 100 μg and the third dose being 50 μg.
In the test, subjects were divided into 1:1 groups and received the first booster shot of the original vaccine (377 people) and a booster shot of the bivalent vaccine mRNA-1273.214 (437 people), with a dose of 50 μg.
mRNA-1273.214 contains 25 μg of original vaccine and 25 μg of Omicron vaccine.
Research result:
1. The 50 μg booster dose of mRNA-1273.214 was well tolerated, and the safety and responsiveness were similar to those of the mRNA-1273 50 μg booster dose.
2. All primary and major secondary immunogenicity goals have been met:
- ①28 days after the booster dose, compared with the prototype mRNA-1273 (50 μg), mRNA-1273.214 (50 μg) produced a better neutralizing antibody response to Omicron;
- ②28 days after the booster dose, mRNA-1273.214 (50 μg) induced a favorable neutralizing antibody response against the original SARS-CoV-2 compared with the original mRNA-1273 (50 μg).
3. mRNA-1273.214 (50 μg) induces potent neutralizing antibody responses against Omicron in all individuals regardless of the previous status of COVID-19 infection.
Specifically, compared with existing vaccines, the bivalent vaccine design using the Omicron/original strain as a booster induces a better immune response against Omicron, while an immune response against the original strain Immune responses of similar intensity were also shown.
In addition, the researchers found that the level of neutralizing antibodies to Omicron in the people who received the modified vaccine was 1.75 times higher than that in the people who received the original vaccine booster.
In addition, Moderna said the booster dose of mRNA-1273.214 was well tolerated, with side effects comparable to the mRNA-1273 booster dose at the 50 µg dose level.
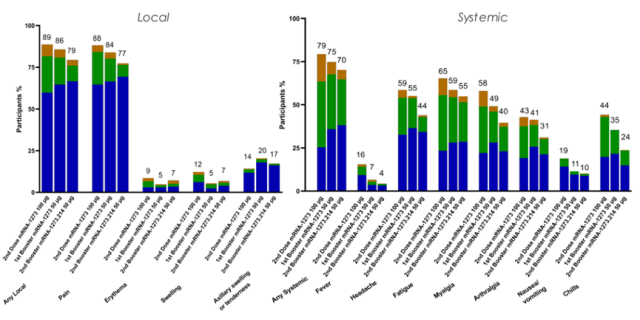
Figure 2 mRNA-1273.214 is safe (Source: [1])
Moderna expects that the neutralizing antibody titers induced by mRNA-1273.214 will be more tolerable over time than the original vaccine, mRNA-1273. Based on the results of the trial, Moderna said it will submit preliminary data from the study to U.S. health regulators in the coming weeks, and hopes to launch a booster shot of the improved version of the vaccine by the end of this summer.
The bivalent design of Omicron superimposed with the original virus strain is likely to become the research and development standard for a new generation of COVID-19 vaccines.
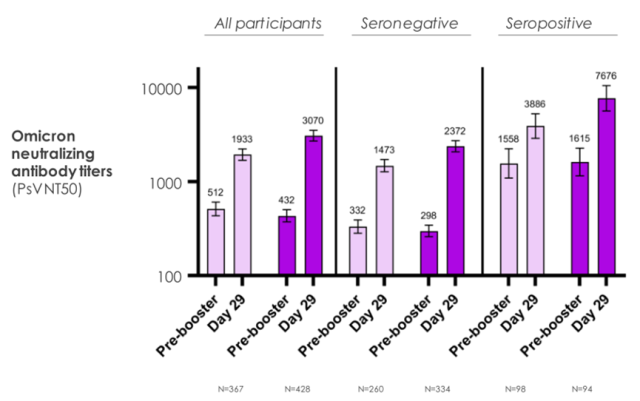
Figure 3 Comparison of neutralizing antibody titers produced by the modified Omicron vaccine with that produced by the original vaccine (Source: [1])
References:
[1] Bivalent COVID Booster Ph 2/3 Interim Analysis (mRNA-1273.214)
Clinical results of Moderna’s Omicron vaccine are more broad-spectrum than original vaccine
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



