BMJ: Patients with cervical precancerous lesions can also get HPV vaccine!
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
BMJ: Patients with cervical precancerous lesions can also get HPV vaccine!
- Red Yeast Rice Scare Grips Japan: Over 114 Hospitalized and 5 Deaths
- Long COVID Brain Fog: Blood-Brain Barrier Damage and Persistent Inflammation
- FDA has mandated a top-level black box warning for all marketed CAR-T therapies
- Can people with high blood pressure eat peanuts?
- What is the difference between dopamine and dobutamine?
- How long can the patient live after heart stent surgery?
BMJ: Patients with cervical precancerous lesions can also get HPV vaccine! May reduce risk of recurrence of cervical precancer after surgery.
The human papillomavirus (HPV) vaccine has been in short supply since it was launched in China, and it is undoubtedly an Internet celebrity vaccine.
There are almost no female friends around who don’t know about this vaccine. When meeting and gathering, they often ask each other, have you been vaccinated?
However, some friends have not been vaccinated against HPV because they have been infected with HPV and have cervical lesions. Do they not know that HPV vaccine is still useful?
Just recently, a multinational research team led by Professor Maria Kyrgiou of Imperial College London School of Medicine published a meta-analysis in the British Medical Journal (BMJ) [1], and their findings provided a reference answer to the above questions.
In this analysis, they found that for patients with HPV infection and grade 2+ cervical intraepithelial neoplasia (CIN) who underwent surgery, HPV vaccination after surgery was associated with a 57% lower risk of recurrence.
Especially when the infected HPV is two high-risk types 16 and 18, the risk of recurrence can be reduced by 74% .

Cervical cancer is the fourth most common female cancer in the world in terms of incidence and mortality [2].
The emergence of HPV vaccine is a milestone event on the road of cervical cancer prevention and treatment.
Existing clinical research evidence fully shows that vaccination is the best way to prevent HPV infection, and the benefit of HPV vaccination is greatest before sexual intercourse [3].
At the same time, another important means to eliminate cervical cancer is the early detection and early treatment of cervical precancerous lesions.
Cervical precancerous lesions, or CIN, can be classified as mild (CIN1), moderate (CIN2), or severe (CIN3).
Clinically, CIN above moderate is also called high-grade CIN. Most of CIN1 will subside naturally, and follow-up is enough; however, CIN2+ may develop into cervical cancer, which requires surgical treatment, and these patients are susceptible to HPV, and they are easy to re-infect even after surgical treatment .
Although compared with younger age groups, HPV vaccination after the age of 26 has higher costs and lower benefits [4], for this HPV-susceptible female population, HPV vaccination is likely to reduce the risk of cervical cancer [5-6] ].
Unfortunately, the existing related studies have not obtained consistent answers, and the sample size of some studies is too small, and the reliability of the conclusions is limited.
In view of this, the research team led by Professor Maria Kyrgiou conducted a search and systematic analysis of the existing literature to clarify the impact of HPV vaccination during surgical treatment on the risk of HPV infection and the risk of HPV infection-related CIN recurrence.
Through literature searches, clinical trial data collection, and screening and exclusion, the researchers ultimately included 18 studies for inclusion in the meta-analysis. Specifically, 2 randomized controlled trials, 12 observational studies (6 retrospective and 6 prospective studies) , and 4 randomized controlled trials post hoc analysis were included.
At the same time, during the analysis, the researchers also used the risk of bias assessment tools RoB-2 and ROBINS-I to evaluate the above studies, and graded the included studies according to the GRADE evidence classification method.
Through the risk of bias assessment and GRADE evidence classification, the quality and level of evidence of the included studies can be comprehensively assessed, so that the interpretation of the analysis results can be more objective.
In this meta-analysis, the researchers used 16 randomized controlled trials and observational studies for the primary outcome analysis and 4 randomized controlled trials post hoc analysis for the secondary outcome analysis.
Results of the primary analysis showed that vaccination at the time of surgery was associated with a 57% lower risk of CIN2+ recurrence in women compared with no HPV vaccination (11 studies, 19,009 participants, median follow-up 36 months) .
Since HPV16 and HPV18 are the two high-risk HPV types with the highest clinical infection rates, the researchers also analyzed the situation of these two HPV types. RESULTS: Compared with no vaccination, HPV vaccination at surgery was associated with a 74% lower risk of HPV16 or HPV18-related CIN2+ recurrence in female patients (6 studies, 1879 participants) ; at the same time, HPV16 or HPV18-related CIN1+ recurrence was associated with a reduced risk 75% relevant (but only involved 1 study with 178 participants) . However, in the above analysis, according to the GRADE classification, the reliability of the included studies is not high.
In addition, HPV vaccination at the time of surgery was associated with a 72% reduction in the risk of CIN3 recurrence in women (questionable vaccination status, 3 studies, 17 757 participants) .
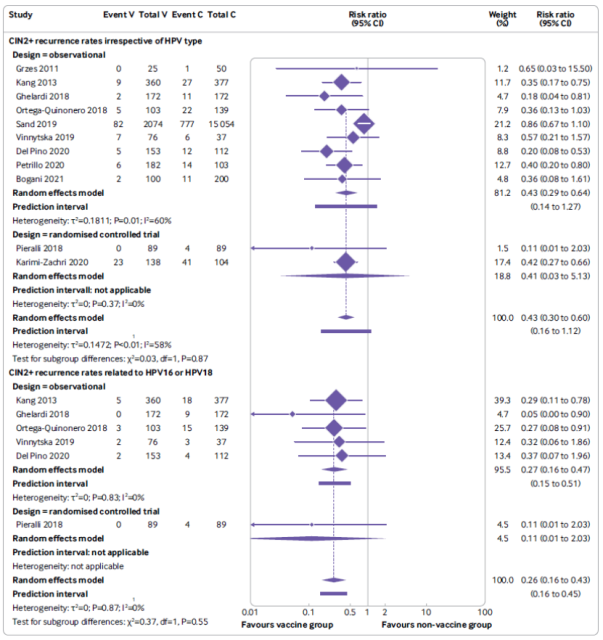 Meta-analysis forest plot of the effect of HPV vaccination after surgery (V) on the risk of recurrence of CIN 2 or CIN 2+ compared with the unvaccinated control group (C);
Meta-analysis forest plot of the effect of HPV vaccination after surgery (V) on the risk of recurrence of CIN 2 or CIN 2+ compared with the unvaccinated control group (C);
the results of the analysis without considering the HPV type (top panel);
Analysis results related to HPV 16 and HPV18 (bottom panel) (analysis results based on randomized controlled trials and observational studies)
The researchers also performed subgroup analyses for factors such as geographic distribution, study protocol, vaccine type (both bivalent and tetravalent), and timing of vaccination (at the time of surgery, or after surgery), and found that these factors did not contribute to the primary analysis.
A clear effect, that is, the reduction in the risk of CIN2+ recurrence after local surgical treatment by HPV vaccination was not affected by the above-mentioned factors .
For other HPV infection-related diseases, including grade 2 or higher intraepithelial neoplasia in sites such as the cervix, vulva, or vagina, compared with unvaccinated HPV vaccine, vaccinated and female patients received local therapy for grade 2 or higher epithelium associated with a 44% lower risk of recurrence of endotumor disease (vaccination status questionable, 2 studies, 296 participants) .
In addition, only one study assessed the effect of vaccination on the risk of developing vulvar or vaginal intraepithelial neoplasia of grade 1 or higher in women following CIN2+ surgery, but no difference was found between the vaccinated and non-vaccinated groups.
Another study evaluated the effect of vaccination and topical treatment of anal intraepithelial neoplasia on the risk of recurrence of high-grade anal intraepithelial neoplasia in men, but also found no difference compared with the unvaccinated population.
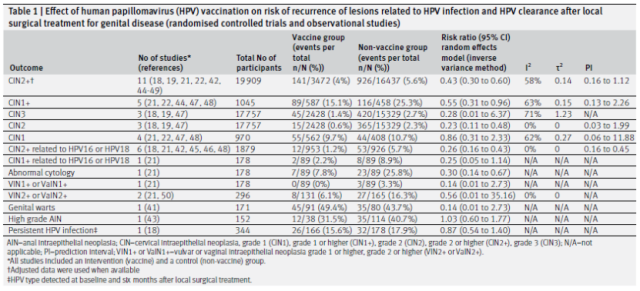 Main results: Effect of HPV vaccination after local surgery on the risk of recurrence of HPV-related lesions and HPV clearance (based on analysis of randomized controlled trials and observational studies)
Main results: Effect of HPV vaccination after local surgery on the risk of recurrence of HPV-related lesions and HPV clearance (based on analysis of randomized controlled trials and observational studies)
Secondary results obtained from post hoc analyses indicated that HPV vaccination was associated with a 55% reduction in the risk of CIN2+ recurrence compared with no vaccination (vaccination status in doubt, 4 studies, 2268 participants, median follow-up 27 months) ) ; was associated with a 36% lower risk of CIN1+ recurrence (3 studies, 1958 participants) , similar to the primary outcome. In contrast to the primary outcome, HPV vaccination with topical therapy did not reduce recurrence of female vulvar or vaginal 1 or 1+, and 2 or 2+ grade intraepithelial neoplasia.
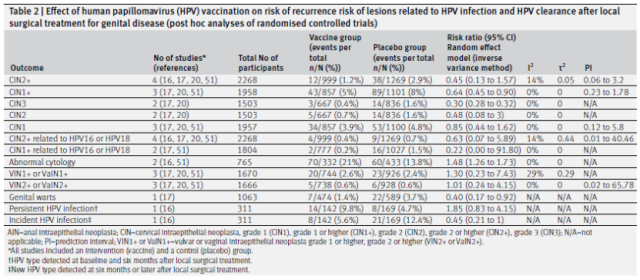 Secondary Outcomes: Effect of HPV Vaccination Following Local Surgery on Risk of HPV-Related Lesion Recurrence and HPV Clearance (Results Based on Post-hoc Analysis)
Secondary Outcomes: Effect of HPV Vaccination Following Local Surgery on Risk of HPV-Related Lesion Recurrence and HPV Clearance (Results Based on Post-hoc Analysis)
Overall, the results of this meta-analysis suggest that HPV vaccination with or after local surgery may reduce the risk of CIN recurrence, especially those associated with HPV16 or HPV18.
However, at the same time, the researchers emphasize that this analysis also has many limitations.
For example, multiple studies included in the analysis used different diagnostic methods, different HPV vaccines, differences in the length and mode of follow-up, and the timing of vaccination, all of which would affect the accuracy of the analysis results;
only 2 quality comparisons were included in the analysis. High RCTs, while more results are mainly from observational studies at risk of bias. Most of these observational studies did not provide the mean age of the participants and did not control for factors such as smoking that could contribute to high relapse rates.
It can be seen that the quality of the existing related research is generally not high.
In the future, a large-scale, high-quality randomized controlled trial is needed for the female population with HPV infection-related diseases, so as to further clarify the HPV vaccination in this population after surgery. effectiveness and cost benefit.
The good news is that a related randomized controlled clinical trial (registration number NCT03979014) is currently underway.
This clinical trial plans to recruit 1000 women aged 18-55 with high-grade CIN as subjects, and only the surgery group is used as a control to study whether vaccinating patients with 9-valent HPV vaccine at the same time as surgery can improve the HPV clearance rate, so as to further Reduction of HPV infection and recurrence of high-grade CIN.
If successful, this clinical trial, with beneficial results from vaccination, could facilitate the implementation of a post-surgical vaccination policy and would make a major contribution to cervical cancer elimination plans for decades to come.
At the same time, HPV vaccination may also benefit patients with other diseases associated with HPV infection, multifocal disease, and other HPV-related malignancies.
References:
1. Kechagias KS, Kalliala I, Bowden SJ, et al. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: systematic review and meta-analysis[J]. BMJ, 2022, 3;378:e070135.
2. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3):209-249.
3. Schiller JT, Lowy DR. Understanding and learning from the success of prophylactic human papillomavirus vaccines[J]. Nat Rev Microbiol, 2012,10(10):681-92
4. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices[J].Wiley Online Library, 2019, 3202-6.
5. Hildesheim A, Gonzalez P, Kreimer AR, et al. Impact of human papillomavirus (HPV) 16 and 18 vaccination on prevalent infections and rates of cervical lesions after excisional treatment[J]. Am J Obstet Gynecol, 2016, 215: 212.e1-15.
6. Joura EA, Garland SM, Paavonen J, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data[J]. BMJ, 2012, 344:e1401.
BMJ: Patients with cervical precancerous lesions can also get HPV vaccine!
(source:internet, reference only)
Disclaimer of medicaltrend.org
Important Note: The information provided is for informational purposes only and should not be considered as medical advice.



