The Past Current status and Future of Endotoxin Testing
- A Single US$2.15-Million Injection to Block 90% of Cancer Cell Formation
- WIV: Prevention of New Disease X and Investigation of the Origin of COVID-19
- Why Botulinum Toxin Reigns as One of the Deadliest Poisons?
- FDA Approves Pfizer’s One-Time Gene Therapy for Hemophilia B: $3.5 Million per Dose
- Aspirin: Study Finds Greater Benefits for These Colorectal Cancer Patients
- Cancer Can Occur Without Genetic Mutations?
The Past Current status and Future of Endotoxin Testing
The Past Current status and Future of Endotoxin Testing. Endotoxin is lipopolysaccharide (LPS) encased in the outer membrane of Gram-negative bacteria.
What is endotoxin?
Endotoxin is lipopolysaccharide (LPS) encased in the outer membrane of Gram-negative bacteria. They represent the most important pyrogens for pharmaceutical and medical device companies. Endotoxins are important because they are ubiquitous in the environment, are thermally stable and cannot be removed by filtration.
Importantly, they are extremely pyrolyzed. Endotoxin stimulates monocytes and macrophages to secrete a series of inflammatory cytokines, including tumor necrosis factor α (TNFα), interleukin (IL)-1 family, IL-6, IL-8, IL-10 family , IL-12 family, IL-15 family and transforming growth factor β (transforming growth factor-β, TGFβ).
Generally, LPS has three structural components. Lipid A, hydrophobic part of LPS, hydrophilic heteropolysaccharide core region and O type specific oligosaccharide region, they differ from strain to strain in serotype. The first sugar in the core region (3-deoxy-D-mannose caprylic acid or KDO) is linked to the 6’position and the core polysaccharide is linked to lipid A. All the biological activities of LPS reside in one area of lipids.
Lipid A has a β-glucosamine backbone with phosphoryl groups at positions 1’and 4′. For E. coli and other members of the Gram-negative bacteria Enterobacteriaceae, this backbone is acylated with β-hydroxymyristate at the 2, 3, 2′, and 3’positions. The secondary acyl groups are esterified with laurate and myristate at the 2’and 3’positions, respectively. The lipid A structure described here is a classic structure and one of the most powerful activators of natural immune response.
Not all gram-negative organisms produce lipid A structures that activate the innate immune response. Activation depends on the presence of phosphoryl groups, the number of acyl chains and the length of acyl chains. LPS from Helicobacter pylori, Yersinia pestis and Francis tularensis are pathogenic, but their LPS structure is difficult to recognize by Toll-like receptors (TLR4), so they can evade host detection.
Even in the Enterobacteriaceae family, the classic LPS architecture will change. Under the conditions of low magnesium and low pH, the addition of palmitate and the modification of the phosphate group of glycolipid A with cationic sugar or phosphoethanolamine occur. Modifications also occur in the environment of high iron, aluminum and calcium.
LPS from all gram-negative bacteria is continuously modified or remodeled. The remodeling of lipid A depends on environmental conditions and is mainly controlled by a two-component adjustment system of PhoP-PhoQ and PmrA-PmrB.
The outer membrane of gram-negative bacteria is highly asymmetric, the inner leaflet contains glycerophospholipid, and the LPS exposed on the cell surface contains LPS. It serves as a penetration barrier. Most nutrients cross this barrier through the outer membrane protein (OMP) family called porins. Changes in the outer membrane will affect its integrity, fluidity and permeability. The remodeling of LPS and outer membrane components represents a coordinated way of survival. Outer membrane vesicles (OMV) have the secretion process of Gram-negative bacteria, which can promote the remodeling of the outer membrane.
Outer membrane vesicles are nanoparticles commonly produced by Gram-negative bacteria. They range from 20-250nm in diameter and contain proteins, lipids, LPS and other biologically active substances that are no longer needed or harmful to survival.
They usually have a similar composition to the bacterial outer membrane of elaborate OMV. OMV production is a stress response of bacteria, which plays an important role in nutrient acquisition, biofilm development, horizontal genetic exchange, and other interspecies interactions that occur in complex microbial communities. These areas are often under temperature and nutritional pressure.
The endotoxins of these microbial communities cannot be produced in the laboratory because of seasonal and other local factors that give local characteristics. These microbial populations are present in all pharmaceutical operations.
Past
In 1912, Hort and Penfold developed and perfected a rabbit animal model, which promoted the research of injection fever. They carefully controlled the breed, gender, weight, food and environmental feeding conditions of the rabbit model. The dose was reported in each experiment.
Take temperature measurements at 30-minute intervals. The experimental design allowed them to finally prove that there is no fever body (FPB) when fresh distilled water is used for intravenous injection. Hort and Penfold canceled all theories related to: (a) water heat, (b) salt heat, (c) carbohydrate heat, (d) fermentation heat, and (e) tissue heat. Unfortunately, it was not until 1923 that their work was properly recognized.
In 1923, Florence Seibert recognized the basic value of Hort and Penfold’s scientific research. Her research on injection fever used the same rabbit model they developed. Seibert successfully confirmed and expanded the prerequisites that the heat source materials in distilled water are of bacterial origin, and these materials will not be removed by filtration.
Most importantly, she introduced “a method for preparing non-heat source water.” Her method of preparing non-pyrogenic distilled water involves heating the connecting stopper several times with alkali (to depyrogenate) and introducing a “collector” above the distillation flask. Capture and prevent pyrogens from being mechanically carried into the distillate.
With the rise of the entire parenteral industry in the 1930s and the large demand for parenteral solutions before and during World War II, the need to ensure that commercial parenteral solutions are not contaminated by pyrogens triggered the development of the United States Pharmacopoeia (USP), pyrogens Pharmacopoeia testing came into being.
In 1941, USP, NIH, FDA, and 14 pharmaceutical companies collaborated on the first study of pyrogen testing. The study used a rabbit model developed by Hort, Penfold, and Siebert. The result of the collaboration resulted in the inclusion of the first pharmacopoeial pyrogen test in the 12th edition of the USP in 1942. The test requires intravenous injection of the solution and a group of rabbits to measure the rectal temperature for three hours.
Between 1964 and 1968, Levin and Bang reported that gram-negative bacterial endotoxins had the ability to coagulate the blood of Atlantic horseshoe crabs. They determined that the sensitivity to bacterial endotoxins in the blood (derived from Gram-negative bacteria) is due to the enzymes in their blood cells, amebocytes.
Since the test is based on horseshoe crab (Limulus sylvestris), and since the enzyme is obtained from limulus cells with high purity by lysing distilled water, the test is rated as limulus amebocyte lysate, or LAL. An article published in 1971 by Levin and Cooper reported that compared with RPT, LAL (limulus reagent) is more sensitive and less expensive. It has stimulated worldwide interest in determining the applicability of BET as an alternative to RPT.
In 1973, the FDA announced the proposed manufacturing specifications for the production of LAL. The manufacturer of LAL must submit each batch of samples to the FDA for testing before use. The temporary use of LAL allows the industry to gain practical experience. The innovative LAL test is routinely used to test raw materials, in-process samples, first batch (FOB) and in-batch (MOB) samples. End of batch (EOB) samples require rabbit testing because EOB is considered the worst case.
Recognizing the simplicity and durability of LAL testing, Baxter Travenol, the largest medical device and LVP manufacturer, made a company decision in 1973 to validate LAL for endotoxin testing at its global facilities.
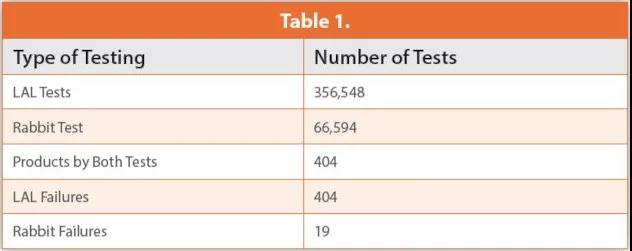
In 1979 and 1982, they reported their annual endotoxin test results. These data include the 66594 USP rabbit pyrogen test and the 356548 LAL test. It is worth noting that both methods tested 404 naturally contaminated samples. The LAL test confirmed the presence of endotoxin, and all 404 samples failed the test. However, according to USP Chapter <151> Pyrogen Test, eight rabbit tests performed on all 404 samples resulted in only 19 failures.
These data clearly indicate that LAL is a more sensitive method and that Gram-negative bacterial endotoxin is the pyrogen that has the greatest impact on the manufacture of parenteral products. These data and 45 years of experience prove the specificity of the LAL test (the ability to detect a range of endotoxins). As shown in Table 1 below.
Current Status
Commercial recombinant protein is being developed to replace the natural source LAL reagent currently defined in the Pharmacopoeia. All three products are based on factor C, which is the first endotoxin binding factor in the LAL cascade. A recombinant product containing all three clotting factors from Asian (Tachypleus) and American (Limulus) horseshoe crab species is also being developed.
The research aimed at proving that the current pharmacopoeia method is equivalent to the recombinant method is largely based on the comparative test of purified LPS or the comparison of clean samples of water for injection (WFI). However, these studies have not solved the specificity problem, that is, the ability to detect multiple natural environmental endotoxins.
Several studies have used environmental endotoxins to compare rFC with LAL. Thorne and others inspected air samples from 10 livestock production facilities. The research design, sample size and statistical evaluation are excellent.
However, air samples from livestock facilities do not represent the pharmaceutical production environment. In addition, the endotoxins associated with bacteria in this sample are more likely to represent the intestinal tract, rather than the non-fermentation tanks that normally reside in the water system. Kikuchi did check for environmental endotoxins from various water sources. The sample set is very limited, but despite this, the data suggests that the recombinant method may underestimate the endotoxin concentration in naturally contaminated water.
Akers et al. Recently, all current literature comparing rFC and LAL was compared. Their review concluded that all the currently available data proved the applicability of rFC, but did not prove comparability. They noticed that most of the published studies that claim to be comparable include data from test articles that have no measurable autoendotoxin activity at any stage of the manufacturing process. When the tested impurity (in this case, endotoxin activity) in the test article is at a quantifiable level, comparability cannot be claimed. The recovery rate of the analyte (RSE or CSE) has not experimentally confirmed the ability of alternative methods to recover natural product contaminants.
Charles River recently completed an evaluation that simultaneously examined three commercially available recombinant Factor C products, a single recombinant LAL cascade under development, and two FDA-approved LAL reagents. Pharmaceutical pretreatment water samples from European parenteral manufacturing facilities were used to evaluate the specificity of the recombinant reagents. This study used a statistically relevant sample set.
It proves that all recombinant reagents may underestimate the concentration of natural endotoxin. The underestimation was not due to the dextran deviation of the LAL reagent, because carboxymethylated curdlan was used as the dextran blocker of Charles River LAL reagent, and both LAL reagents produced similar results. In addition, the results of this study indicate that all recombinant products require further development to detect fever bodies (endotoxins) identified by Hort, Penfold, and Seibert 108 years ago.
Future
The ability to measure and detect all endotoxins (Burkholderiaceae, Methylobacteriaceae, members of the Comamonadaceae class/family) produced by the microbial communities living in the purified water system is absolutely necessary for the operational control of the WFI system.
Currently, the restructuring alternatives tested by LAL cannot do this consistently. Further progress must be made in recombinant protein chemistry or recombinant formulations. The refining of LAL recombinant substitutes must consider the reactivity to natural environmental endotoxins, rather than highly purified lipopolysaccharide standards. The difference between the pharmacopoeial LAL test and any recombinant alternative must be resolved scientifically.
Similar to the LAL test in the early 1980s, LAL is not a single enzyme, and LAL is not three enzymes. LAL does contain three protease zymogens, which are known to be involved in the sequential activation of bacterial endotoxins. LAL also contains factor G enzymes that are sensitive to β1-3 glucan. LAL contains soluble protein coagulogen, which can be converted into insoluble gel by activated thrombin.
LAL also contains three serine protease inhibitor-like proteins, which can regulate the activation cascade of LAL endotoxin and dextran. LAL contains anti-LPS factors, which can neutralize bacterial endotoxins, antimicrobial peptides, α2-macroglobulin, large defensins and cystatin.
LAL is the original immune system of horseshoe crabs for 4.5 million years, and human horseshoe crabs will continue to serve as an effective and reliable safety test for human and animal injections. We recognize that there are concerns about the sustainability of the Atlantic horse population.
Charles River developed an ammunition-based microfluidic LAL test that can reduce LAL resources to 95%. Charles River and all FDA-approved LAL manufacturers have developed conservation and education programs for decades to ensure the protection of this outstanding species
(sourceinternet, reference only)
Disclaimer of medicaltrend.org



