Odevixibat: Treat progressive familial intrahepatic cholestasis
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Odevixibat: Treat progressive familial intrahepatic cholestasis
Odevixibat: Treat progressive familial intrahepatic cholestasis. For the treatment of cholestasis, odevixibat applies for listing in the United States and Europe!
Recently, Albireo Pharma announced that it has submitted a New Drug Application (NDA) to the US Food and Drug Administration (FDA) and a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) to seek approval for odevixibat For the treatment of progressive familial intrahepatic cholestasis (PFIC). PFIC is a devastating disease, and the only option for many patients is liver transplantation or other invasive surgery.
EMA will review odevixibat through an accelerated approval process. With the completion of the FDA and EMA regulatory application documents, odevixibat has the potential to become the first therapeutic drug approved for PFIC in the United States and Europe. Previously, EMA has granted odevixibat the Orphan Drug Designation and Priority Drug Designation (PRIME) for the treatment of PFIC, and the FDA has granted odevixibat the Fast Track Designation (FTC), Rare Pediatric Disease Designation (RPDD) and Orphan Drug Designation (ODD) for the treatment of PFIC.
odevixibat is a pioneering, potent, selective, non-systemic, ileal bile acid transporter (IBAT) inhibitor, with minimal systemic exposure and acting locally in the intestine. At present, the drug is being developed for the treatment of rare childhood cholestatic liver diseases, including PFIC, biliary atresia, and Alagille syndrome; among them, PFIC is the first target indication.
Data recently announced at the AASLD meeting confirmed that odevixibat treatment can provide durable relief for patients with PFIC. The global Phase 3 PEDFIC-1 clinical study is the largest study ever conducted in PFIC. The results showed that the study met the primary endpoint of the US and EU regulatory requirements: compared with placebo, odevixibat significantly reduced bile acid response (SBA, p=0.003), significantly improved skin itching (p=0.004), and the rate of diarrhea was only For single digits.
In addition, long-term data from PEDFIC-2 (open-label phase 3 extension study) showed that in patients treated for up to 48 weeks, SBA was continuously and persistently reduced, pruritus evaluation improved, and liver and growth function indicators were encouraging. In these two studies, odevixibat was well tolerated, and the adverse events (TEAE) caused by treatment were mostly mild or moderate.
Overall, these studies have once again confirmed that odevixibat has the potential to become the first drug approved for the treatment of PFIC. PFIC is a devastating disease and is currently treated with surgery including liver transplantation. Ovidixbat is a safe and effective treatment drug, which has the potential to bring real changes to PFIC patients and their families. After obtaining regulatory approval, Albireo plans to launch odevixibat on the market in the second half of 2021.
PEDFIC-1 is a randomized, double-blind, placebo-controlled, global multicenter phase 3 study, conducted in 62 patients with PFIC1 or PFIC2 aged 6 months to 15.9 years. In the study, patients were randomly assigned to receive two commercial oral doses of odevixibat (40μg/kg/day, 120μg/kg/day) or placebo, once a day for 24 weeks. Patients in the odevixibat treatment group received oral capsules or sprays once a day. These two types of preparations do not require refrigeration.
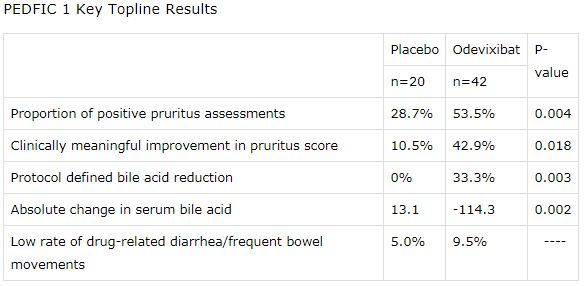
The data showed that in the main analysis, the study reached the primary endpoint in line with US regulations: the positive rate of pruritus assessment in the odevixibat treatment group was 53.5%, and the placebo group was 28.7% (p=0.004). In terms of secondary endpoints, 42.9% of patients in the odevixibat treatment group had a clinically significant improvement in the pruritus score (defined as: on the 0-4 subscale, the pruritus score decreased by ≥1.0 points from the baseline at week 24), and the placebo group was 10.5 %(P=0.018).
In addition, the study also reached the primary endpoint in compliance with EU regulations: 33.3% of patients in the odevixibat treatment group reduced serum bile acid (sBA) by 70% or reached 70 μmol/L levels, while no patients in the placebo group achieved this goal (p= 0.003). In terms of secondary endpoints, bile acids in the odevixibat treatment group decreased by an average of 114.3 μmol/L, and the placebo group increased by 13.1 μmol/L (p=0.002).
The two doses of odevixibat were statistically significant in each end point. In the study, odevixibat was well tolerated. The incidence of adverse events was similar to that of placebo. During the study, there were no serious adverse events (SAE) related to drugs. Diarrhea/frequent bowel movements were the most common treatment-related gastrointestinal adverse events, which occurred in 9.5% of patients treated with odevixibat and 5.0% in the placebo group.
Disclaimer of medicaltrend.org



