Bispecific antibody molecular construction
- Normal Liver Cells Found to Promote Cancer Metastasis to the Liver
- Nearly 80% Complete Remission: Breakthrough in ADC Anti-Tumor Treatment
- Vaccination Against Common Diseases May Prevent Dementia!
- New Alzheimer’s Disease (AD) Diagnosis and Staging Criteria
- Breakthrough in Alzheimer’s Disease: New Nasal Spray Halts Cognitive Decline by Targeting Toxic Protein
- Can the Tap Water at the Paris Olympics be Drunk Directly?
Bispecific antibody molecular construction
Bispecific antibody molecular construction. In the past 20 years, therapeutic bispecific antibodies (double antibodies) have developed rapidly. The “double antibody family” has as many as 100 structural forms, including combinations of different species. The simplest double antibody molecule consists of two small molecules (antibody heavy chain and light chain variable region sequence) that can specifically bind to antigen sites respectively.
There are also IgG-like molecules and dimeric molecules formed by macromolecular complexes that can bind to different antigenic sites. The application of complex molecular design and genetic engineering has solved many technical problems related to the preparation of double antibodies, such as stability, solubility, and other relevant parameters related to druggable antibody molecules. These parameters can be summed up to be the developability of antibody molecules.
In addition, different product development goals determine the characteristics of the bispecific antibodies produced, guide the structure of the bispecific antibodies to be formed, and the molecular size and arrangement of their binding regions may need to be changed during the development process. , Valence, flexibility and geometric structure. Therefore, there is no optimal bispecific antibody structure, and there is no certain structure format that is universally applicable to any bispecific antibody molecule.
On the contrary, the diversity of bispecific antibody structure is a precious resource, and it is possible to achieve a blossoming of therapeutic bispecific antibodies. This article will comprehensively describe most of the bispecific antibody formats.
Introduction
Double antibodies have attracted more and more attention in disease diagnosis and treatment. Double antibodies can simultaneously recognize two different epitopes of the same or different antigens, while natural antibodies can only recognize one epitope. Double antibodies have a relatively long history, starting in the 1960s, when they were able to recombine two different antigen-binding fragments (Fabs) from polyclonal antibodies in serum into bispecific F(ab’)2 molecules. .
The establishment of hybridoma technology in 1975 consolidated and strengthened the concept of bispecific antibodies, which made it possible to produce bispecific antibodies in the true sense. The idea of early bispecific antibody construction was to combine the two Monoclonal antibodies are hinged, or hybrid hybridomas can be produced by fusing two hybridoma cell lines.
With the development of recombinant antibody technology, it is possible to produce bispecific antibodies with specific structure, composition, biochemical properties, functions and pharmacological properties.
Bispecific antibodies are widely used in diagnosis, imaging, disease prevention and treatment. In the beginning, the application of bispecific antibodies in the therapeutic field was mainly focused on the relocation of effector cells to tumor cells, including the relocation of T cells, which cannot be achieved with traditional monoclonal antibodies.
In the past few decades, a variety of therapeutic strategies based on bispecific antibodies have been established. In addition to relocated effector molecules, cell and gene therapy vectors, dual targeting, pre-targeting strategies, prolonged half-life, and methods to cross biological barriers such as the blood-brain barrier have been developed.
Bispecific antibodies are considered to be the most potential treatment for many indications, including cancer, chronic inflammation, autoimmune diseases, neurodegenerative diseases, bleeding diseases, infections and other diseases. The use of these molecules has been widely reported. This article will focus on describing the numerous structures and corresponding strategies for producing bispecific antibodies. Bispecific antibodies are undoubtedly the fastest growing field.
With the continuous emergence of new structural forms, keeping up with the pace is really a challenging task. This article cannot be exhaustive, nor can it cover all the existing dual-resistance structural styles. The exploration of the structure of dual-resistance and the learning of relevant knowledge cannot be accomplished overnight. There is a long way to go.
Classification of bispecific antibodies
Most natural antibodies are bivalent or multivalent molecules directed against the same antigen binding site. Due to the instability of the hinge region of IgG4 molecules, Fab arm exchange usually occurs. This is a random process that produces bivalent molecules that target two specific sites.
Bispecific antibodies are artificial molecules produced against two distinct specific sites, which usually cannot occur in the natural state. This means that knowledge of biochemistry, molecular and genetics is needed to be modified to achieve this. The oldest method is the chemical coupling method to connect two antibody molecules or antibody molecule fragments by force. This article will not go into details (CovX-Bodies) Figure 2-1.
In addition, by fusing the two hybridoma cells, bispecific IgG molecules containing two different heavy chains and two different light chains are produced in the new hybrid-hybridoma cells, as shown in Figure 2-1. Functional molecular by-products.
The difficulty in producing IgG-like bispecific molecules is that the antigen binding site is composed of the variable region sequences (VL, VH) of the light chain and the heavy chain. A bispecific antibody requires two different heavy chains. The two different light chains are composed of an asymmetric form and contain at least two Fv regions.
The free combination of two different heavy chains and two different light chains results in as many as 16 kinds, resulting in 10 different molecular structures, but only one desired bispecific structure. The rest are some non-functional molecules or monoclonal antibody molecules.
For bispecific antibodies, it is indeed a big challenge to directly and powerfully complete the assembly of heavy and heavy chains, and heavy and light chains. For more than two decades, people have been committed to solving this problem through various strategies.
Recombinant bispecific antibodies can be classified according to structure and composition. One major difference is whether the double antibody molecule contains an Fc region. Double antibody molecules without Fc region lack Fc-mediated effector functions, such as antibody-dependent cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), fixation of complement, and FcRn-mediated antibody circulation (will greatly Extend the half-life of IgG).
Bispecific antibodies containing Fc regions can be further divided into IgG-like molecules and IgG-like derivatives that additionally contain other binding sites. Different bispecific molecules can be divided into symmetrical structure and asymmetrical structure according to symmetry. For example, most IgG-like bispecific molecules are asymmetric, while IgG-like fusion proteins are usually symmetrical in composition (Figure 1).
Furthermore, their characteristics can be distinguished based on the number of binding sites. In a simple design, like an IgG molecule, a bispecific antibody contains two binding sites that can target their respective epitopes, ie, the “1+1” format, which is bivalent.
On the basis of IgG, additional binding sites are added to each chain to form a 4-valent molecule, which is the “2+2” form. Other forms include the creation of molecular structures such as “1+2” or “1+3”, with one binding site that binds to one epitope of one antigen, and 2 or 3 binding sites to bind to another antigen, respectively. The 3 or 4 valence molecules can be generated by increasing the price point or increasing the specificity. In addition, the number of bispecific antibody chains produced can be diversified.
Therefore, a typical IgG-like bispecific antibody needs to express four different polypeptide chains, but in some structures only 3, 2, or even one chain can be achieved.
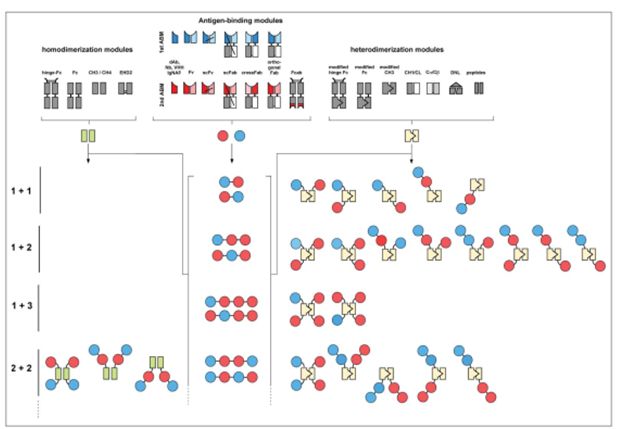
Figure 1 Schematic diagram of modules and examples of producing homologous or heterodimer molecules
A wide range of different modules-antigen binding molecules can be used to produce a schematic diagram of homologous or heterodimeric bispecific antibody molecules (Figure 1), which can be designed according to the target molecule, valence, molecular size, Flexibility, pharmacokinetics and pharmacodynamic characteristics are required to design bispecific antibodies.
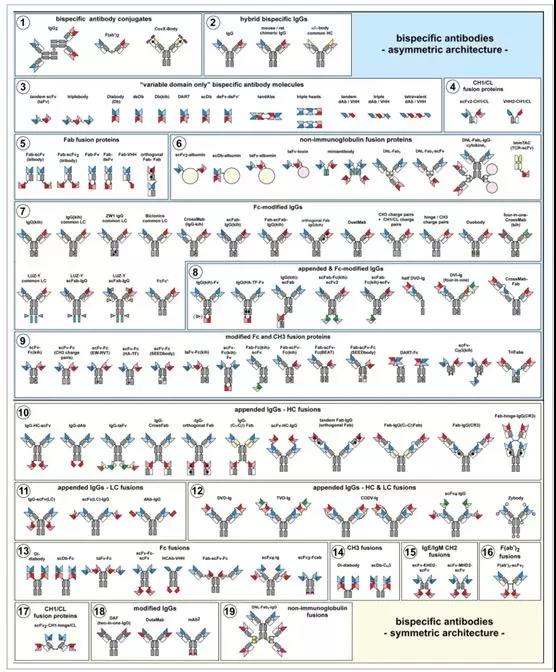
Figure 2. Molecular structure overview of bispecific antibodies
Bispecific antibody format lacking Fc
Tandem connection of single-chain variable region fragments (scFv2, taFv) and trisomy
The simplest bispecific antibody consists of two antigen-binding fragments of antibodies. The single-chain variable region fragment scFv format is derived from VH and VL, represents the smallest antigen binding unit, and is the most extensive bispecific antibody component.
Due to the conformation of scFv, bispecific antibodies can be formed by connecting two scFvs with a linker. Therefore, these molecules are bivalent and monovalent for each antigen, and their typical molecular weight is 50-60KDa.
The first report on tandem scFv molecules dates back 20 years (Figure 2-3). In this study, two scFvs were expressed by a 27-amino acid helical linker or flexible linker (Trichoderma dehydrogenase I).
Since then, many scFv structures expressed in tandem have been reported. In principle, two scFvs in tandem form taFvs, and their sequence is clear (VH-VL or VL-VH). The length and composition of the linker affect the folding, stability, and binding of taFvs to antigen.
Various linkers can be used to connect two scFvs, the shortest linker such as Ala3, the hydrophilic linker screened by phage display, the linker rich in Gly-Ser, the linker in helical conformation and Some molecules derived from immunoglobulin and non-immunoglobulin.
It consists of 5 and 15 amino acid long Gly-Ser linkers, which are connected to two scFv fragments that are active against CD3 and CD19, respectively, to form two bispecific antibodies with different linkers. In vitro experiments show that there is a biological There is no difference in activity, which means that the scFv linked by the short linker is flexible enough to recruit T cells and cross-link to the antigen displayed by the tumor cells.
Bispecific antibodies with tandem scFv are widely used in tumor immunotherapy to redirect T cells to tumor cells or tumor-related cells in the tumor microenvironment. This structure forms a bispecific antibody molecule (BiTE) that redirects T cells. The first (BiTE) bispecific antibody molecule (Blincyto, blinatumomab) is approved for the treatment of Philadelphia chromosome-negative precursor B-cell acute lymphoblastic leukemia ( In patients with B-cellALL), blinatumomab is composed of CD19 antibody scFv in the VL-VH direction through a G4S linker connected to CD3 antibody scFv arranged in VH-VL.
Due to its small molecular weight, BiTE molecules can be quickly eliminated with a half-life of only 1.25h. The antibody treatment requires continuous intravenous injection with a syringe pump at a constant flow rate, and the treatment lasts at least 4 weeks.
Tandem scFv bispecific antibodies include scFv against CD16 and bispecific antibodies (BiKEs) that redirect NK cells. In one study, anti-CD16 scFv was linked to anti-CD133 antibody scFv by a 20 amino acid linker derived from human muscle aldolase for the treatment of colon cancer.
The construction feature of this modular approach allows expansion of serial scFv, for example, adding more scFv. That is, it is used to produce trivalent antibodies. The bispecific molecule (trisomy) contains two CD19 binding sites and one CD16 binding site to redirect NK cells (Figure 2-3).
All the scFv units in the trisome, the VH and VL domains of the variable domain, all of which are connected in the VL-VH direction, are connected by a 20 amino acid (G4S)4 linker. This method allows the generation of trispecific molecules, just like real trivalent molecules. The trispecific trisomy molecules target CD16, CD19, CD33, and target different antigens on the surface of mixed leukemia cells, redirecting NK cells to the surrounding cancer cells. .
The stability of a single scFv module is a general concern, because the interaction between molecules only occurs between the VH-VL interface, and there is no covalent bond between them. The improvement of the stability of tandem scFv requires the introduction of disulfide bonds in the VH domain and VL domain of the scFv unit (introduction of Cys at VH 44 and VL 100) to produce disulfide bond-stable scFvs (dsFv, dssFv).
This method can also introduce trivalent or even multivalent antibodies to stabilize 1, 2, or even all scFv units. Another method is to introduce hydrogen bonds (VH39, VL38) in the VH and VL domains to replace the VH-VL interaction maintained by electrostatic attraction.
Bispecific domain antibody fusion protein
Arranging antigen binding sites in tandem, single domain antibodies such as VH or VL domains, VHH, VNAR and nano monoclonal antibodies can all be used to construct bispecific antibody molecules, and these methods can also be used for scaffold proteins to produce antibodies Like protein (Figure 2-3).
Containing 2 or more single domain antibodies will produce bivalent, trivalent or more valent molecules in one or more specific molecules. For example, two VHH domains are fused by a long hinge region sequence, and the linker is derived from a sequence or flexible sequence upstream of the hinge region of llama IgG2a that is not easily degraded by proteases. Another example is a flexible linker connecting two shark immunoglobulin neoantigen receptors (VNAR).
The linker consists of amino acids in the hinge region of the natural shark immunoglobulin neoantigen receptor (PGVQPSP), followed by the flexible GGGGSG sequence. In another study, two human single-domain antibodies were combined into the bispecific antibody DAbs, which targeted two antigens of Candida albicans.
For example, in the tetravalent antibody produced in an example, four VHHs are linked into a bispecific VHH fusion protein by a linker, two VHHs target TcdA, and two VHHs target TcdB. (These two antigens are from Clostridium difficile ), the four VHH molecules are connected by three flexible linkers (G3S)4. In vivo and in vitro experiments showed that the fusion protein enhanced the neutralization effect.
Nanobodies can be combined to form a trivalent, bispecific molecule, and then connected to a serum albumin to extend the serum half-life, and the other two sites target the target antigen. A Nanobody fusion protein targeting IL-6R has now entered clinical trials.
Here is a fusion protein consisting of three Nanobodies connected by two GGGGSGGS linkers. In a similar way, the trivalent bispecific VHH fusion protein is used for the treatment of foot-and-mouth disease virus, fused with porcine immunoglobulin, and significantly enhances the in vivo half-life of the fusion protein.
Dimers and dimer derivatives
Diabody (Db) is a bivalent molecule composed of two chains. Each chain contains a VH domain and a VL domain. The VH and VL on the two chains come from two different antibodies. In the dimer form, the two variable region domains are connected by a short linker of 5 amino acid residues, such as GGGGS.
In fact, due to the length of the linker, it is shorter than the linker required for assembly in the scFv chain formed by the antigen binding site. The two chains dimerize in a head-to-tail direction to produce a compact molecule. The molecular structure is similar to a tandem scFv , The molecular weight is about 50Kda. Express two chains in the same cell, one in the VHA-VLB configuration, the other in the VHB-VLA configuration (A and B represent two different specific molecules) or in the VLA-VHB and VLB-VHA configuration Produce heterodimerized bispecific antibodies with correct pairing of variable regions (Figure 2-3).
Bispecific antibodies have been used to redirect effector cells, effector molecules, and other uses. For example, the anti-epidermal growth factor antibody EGFR and the anti-insulin-like growth factor antibody IGF-R constitute a bispecific antibody. The results show that the optimal VH/VL arrangement direction affects the binding of the antibody to the antigen to a certain extent. In addition to the order of the domains, the length and composition of the linker need to be optimized.
Studies have shown that anti-neuraminidase scFv is easy to assemble into a double body when it is connected in two orientations of VH-VL and VL-VH, and the linker is a linker of 3-12 GS. When the linker is shorter, it will Produce trimer, tetramer assembly.
One problem with bispecific dimer molecules is that two different chains, VHA-VLB and VHB-VLA are co-expressed in one cell, and it is easy to produce incorrectly paired variable region domains (VHA/VLB, VHB/VLA ). A further problem is that because the two chains are not connected by covalent bonds, the stability is poor.
One solution is to introduce disulfide bonds between the paired VH-VL domains (dsDb) (Figure 2-3). This method can increase stability. A further improvement of this method is to introduce disulfide bonds in both pairs of VH-VL pairings. In addition, only a 10-amino acid linker is used in the VHA-VLB connection, while VHB and VLA are expressed separately. The dsFv-dsFv’ structure of the three polypeptide chains (Figure 2-3). In addition, you can also try a new model for different domains to form heterodimers.
For example, a knob-into-hole bispecific double body is formed (Figure 2-3). An example of this method is a bispecific double body composed of anti-epidermal growth factor receptor HER2 and anti-CD3 antibody. This structure introduces mutations at a different site between a paired VH and VL, which significantly increases the formation of heterodimers. Of course, in some cases, such a mutation form is likely to affect the binding of the antibody to the antigen, which means that the mutation site must be carefully selected.
Another alternative is to form a single chain and change the method of forming a heterodimer dimer. The first chain (VHA-VLB or VLA-VHB) and the second chain of the dimer will be formed. (VHB-VLA or VLB-VHA) are connected by 15 amino acid flexible linkers. In this single-stranded dimer molecule (scDbs), the long linker ensures the correct assembly of the two chains and improves the stability of the molecule without changing the antigen binding activity.
scDb is composed of four variable region domains connected by three linkers, two flanking linkers and one middle linker. Later, the linker was also used in other scFv assembly. Some researchers have used phage library screening to optimize the length and amino acid composition of the linker.
Studies have found that during the formation of single-stranded duplexes, the length of the two flanking linkers is 2-6 amino acids is the most appropriate, and the middle linker is more than 13 amino acids in length. Other suitable intermediate linkers include the alpha helix sequence derived from the La C-terminal domain of the nucleoprotein.
The single-chain duplex scDb is sometimes used to generate tetravalent bispecific antibodies. This can be achieved by shortening the middle linker so that the two scDb polypeptide chains form a head-to-tail dimer molecule. TandAb (Figure 2-3) has a molecular weight of about 100KDa, twice the molecular weight of scDb.
In TandAb composed of CD19 and CD3 antibodies, the intermediate linker uses 6-12 amino acids. This method of generating TandAb tetravalent has also been successfully used to redirect the TandAb tetravalent antibody composed of NK cell CC16A antibody and CD33 antibody. In this structure, three linkers consisting of 9 amino acids and 3 GGS repeats are used.
The TandAb (AFM13) has been used in clinical phase 1 trials for the treatment of relapsed or refractory Hodgkin’s lymphoma. In another TandAb structure, the three linkers used GGSG, GGSGG, GGSGGS for the antibodies against CD33 and CD3.
Regarding the formation of dimers, another way is to add Cys to the C ends of the two chains. The diabody formed by this method is called dual affinity redirection protein (DART) (Figure 2-3). The first example of this structure is for two molecules, CD32B and CD16.
The VL domain and VH domain of each chain are connected by 8 amino acids GGGSGGGG. The C-terminus of the first chain is extended by the upstream sequence FNRGEC or LGGC derived from the IgG1 hinge region, and the C-terminus of the second chain is extended by VEPKEC (source At the C-terminus of κ chain) or LGGC for extension.
All modifications produce stable, disulfide-linked molecules, and maintain the corresponding antigen-binding activity. This structure is also used to construct CD19 X CD3 bispecific antibodies.
The use of dimers to produce tetravalent bispecific molecules has been described before. In one example, an additional VHA was fused to the N-terminus of VLA-VHB and co-expressed with the polypeptide chain VLA-VHA-VHA, using 16 amino acid residues rich in GS to connect the additional variable region to the dimer At the core (Figure 2-3). In this study, the quadrivalent bispecific form is composed of VHA-VHA-VHB and VLB-VLA-VLA, all VH domains are on one chain, and all light chain VL structures And presented on another chain.
The C-terminus of the first two domains of each chain is connected by a linker of 16, 14 amino acids, and the first two domains are linked by a GGGGS linker. Both three-head molecules (BS6, BS8) can be expressed in E. coli to produce active molecules, which may be used to deliver radiolabeled divalent haptens to tumor cells.
Fab fusion protein
Bispecific antibodies lacking Fc can also be constructed on the basis of Fab and fused with other units. Fabs are composed of a light chain and a heavy chain fragment, Fd, to produce bivalent, bispecific molecules. It is also possible to fuse scFv at the C-terminus of the light chain or Fd to produce a trivalent, bispecific, and tri-specific fusion protein (bibody Fab-L-scFv, Fab-HscFv), even at the C-terminus of both chains scFv produces tribody (tribody, Fab-(scFv)2) (Figure 2-5).
In another example, the Fd chain contains five upstream amino acids EPSGP in the hinge region, and the scFv is fused to the C-terminus of the L and Fd chains via a DVPSGPG or (G4S)3 linker. This study shows that the VH and VL domains in Fab greatly enhance CH1-CL-mediated heterodimerization and secretion. All forms are fully effective in bispecific binding and are described as having a low aggregation and stability tendency under physiological conditions. For other scFv fusion proteins, the linker connecting the scFv to the L chain or the Fd chain, as well as the L chain, the Fd chain, and the order of the domains connected by the linker on the scFv, can be used to adjust the flexibility of the functional molecule.
For example, trivalent bispecific Fab-scFv and Fab(scFv) 2 fusion proteins have been used for antibodies against CD19 and CD16. Here, the scFv uses the VL-VH sequence. The scFv contains inter-domain disulfide bonds for stabilization and is fused to the Fab part through the GVPGGS linker. In another example, the fusion of a disulfide bond stabilized scFv to the Fab via a (G4S)n linker produces a monovalent binding to cMET.
The fusion of one or two additional binding sites in the Fab creates a bivalent, trivalent molecule. Extending the Fab part to the hinge region will produce a tetravalent F(ab’)2-scFv fusion protein, covalently linked to the hinge region in a symmetrical form (Figure 2-6), such as an anti-dextran/dansyl bispecific antibody. Generally, this method can also be used to fuse single domain antibodies or scaffold proteins to Fab, for example, the Fab-H-VHH fusion protein produced targets HER2 and CD16.
Fabs can also generate strong heterodimerized Fab-like parts by connecting them at the C-terminus with a flexible linking polypeptide (instead of the hinge region). This kind of TriFabs, bivalent, bispecific fusion protein contains three functional Fab units (Figure 2-9). TriFabs contains two regulatory Fabs, as heterodimerization modules, through 20 flexible amino acid peptides Linker (G4S) 4 is fused to produce an asymmetric Fab-like antibody.
Subsequent VH fusion of a CH3 containing a Knob (T366W) mutation and a VL domain fused with a CH3 containing Hole (T366S, L368A, Y407V), through Knob and Hole to complete the pairing. In order to increase stability, inter-domain disulfide bonds are used to stabilize the backbone of the variable region (H44-L100). The whole structure is similar to IgG, except that Fc is replaced by the backbone region.
The lack of disulfide bonds in TriFabs can be further compensated by introducing disulfide bonds in the CH3 domain (S354C-Y349C). Different bispecific TriFabs exhibit different functions, such as bispecific antibodies directed against digoxin or biotin in the stem region, and Fab arms directed against CD33, GPC3 or LeY.
The Fab-Fab fusion protein can be produced by fusing the Fd chain of the first Fab arm with the n-terminus of the Fd chain of the second Fab arm to produce a polypeptide chain composed of VHA-CH1-linker-VHB-CH and express two light chains separately. Chain (Figure 2-5). However, two light chains can randomly interact with Fd chains to produce four different molecules, only one of which is a bispecific molecule.
In order to pair the homologous light chain with the corresponding Fd correctly, some mutations can be introduced. An example of this is the use of orthogonal Fabs to produce bispecific Fab-Fab fusion proteins that target EGFR and CD3, redirecting effector T cells to tumor cells expressing EGFR.
Interestingly, Fab-Fab fusion protein has increased thermal stability compared with scFv linked in series. The tandem scFv has the potential to kill tumor cells. It is very likely that the relatively small molecular weight scFv affects the distance between effector cells and target cells, as well as the immune synapses formed by immune cells.
Fab can also be used as a heterodimerization module to bind the second specific VH and VL domains. This is generally used to generate a Fab-Fv fusion structure. Generally, VH is fused to the C-terminus of the Fd chain, and VL is fused to the C-terminus of the light chain (Figure 2-5).
In order to stabilize the fused Fv structure, a disulfide bond (H44-L100) was introduced between the chains to generate Fab-dsFv molecules. These structures increase the half-life of Fab, directly target the corresponding antigen, and the Fv part can bind to serum proteins. The Fab-dsFv molecule is stable under physiological conditions and retains the affinity for the two antigens. In mice and cynomolgus monkey in vivo experiments, it has shown good pharmacokinetic characteristics, and its effect is similar to PEGylated Fab’.
In addition to the use of Fab, the recombinant T cell receptor TCR, which contains α and β extracellular domains, is assembled into a Fab-like structure that can be combined with MHC-display polypeptide complexes. Recombinant TCR fused with scFv can also be used as a structure to produce bispecific molecules, for example, by fusing scFv with TCR β chain through a flexible linker to construct a bispecific antibody.
These bispecific molecules (ImmTACs) (Figure 2-6) can recognize tumor cells through TCR, and redirect effector T cells by targeting CD3 molecules through fused scFv. To increase the stability of the α and β chain heterodimers of TCR, it is necessary to introduce a disulfide bond at the C-terminus of the α and β chains.
Other fusion proteins lacking Fc
The heterodimerization of the antigen-binding protein scFv lacking Fc requires the help of dimerized polypeptides (Figure 2-6). Heterodimerized polypeptides are diverse, including helix-helix leucine zipper structures, from The dimer “zipper” peptides in Jun and Fos proteins are fused to Fab’ molecules or scFv molecules, and heterodimers are formed by methods such as Jun, Fos homodimer reduction, recombination, and reoxidation. In another method, two different scFvs are connected by a double-helix motif and assembled to form a four-helix bundle, finally producing a tetravalent bispecific molecule.
The CH1 and CL domains of IgG can also be used to form heterodimers, bispecific scFv fusion proteins (Figure 2-4). The scFv molecules targeting EGFR and CD2 form a scFv-CH1/scFv-CL fusion protein heterodimerization molecule through the CH1 and Cκ domains of IgG1. The CH1 and CL domains are covalently linked through natural disulfide bonds to form a stable heterodimer. Choose different lengths, and the flexible linker connects the N-terminal of the scFv to CH1 or CL.
The CH1-CL heterodimerization module further produces a bispecific single domain antibody fusion protein (Figure 2-4), which has been successfully used to redirect the CD16 antibody against NK cells and the CEA antibody targeting carcinoembryonic antigen. Construction of bispecific antibodies. Further extend CH1 with the hinge region sequence to form a scFv-CH1-hinge/scFv-CL heterodimerized protein, resulting in a tetravalent bispecific molecule (Figure 2-17).
This structure is used for the construction of bispecific antibodies targeting HIV gp41 antibody and redirecting neutrophil IgA receptor (CD89) antibody. The bivalent scFv-CH1/scFv-CL fusion protein and the tetravalent scFv-CH1-hinge/scFv-CL fusion protein have observed obvious antibody-dependent cell-mediated viral suppression, but the tandem scFv can be targeted The same antigen, but no virus inhibitory activity was observed.
So far, what we have described is the direct fusion of antigen binding proteins or the use of immunoglobulin derivatives to produce bispecific antibodies with heterodimeric structures. However, non-immunoglobulin-like heterodimer modules can also form heterodimers in covalent or non-covalent forms through different binding sites.
One example is the method called dock-and-lock (DNL), which uses the difference between the regulatory subunit of cAMP-dependent protein kinase (PKA) and the anchoring domain (AD) of the A protease anchor protein. The source dimer completes the assembly strategy. Here, the anchor domain AD consists of 23 amino acids and can be fused to the first binding site, and the second binding subunit is fused with a 44-amino acid dimerized DOCK domain (DDD).
This strategy will form a trimeric complex, that is, a protein fused with AD. Two DDD fusion proteins combine to form a trimer. Disulfide bonds can also be introduced between AD and DDD to allow them to pass covalently. The key is connected. The feasibility of this design to produce bispecific and trispecific antibodies has been demonstrated. The trivalent DNL-Fab3 fusion protein can directly target the cancer beat antigen CEA and histamine succinylglycine (HSG).
Two fusion proteins (DDD is connected to the CH1 C-terminus of the anti-CEA antibody Fab through 14 amino acids, and the other structure is the fusion of the AD domain to the CH1 C-terminus of the anti-HSP antibody Fab), express these two proteins separately, and then Together, it produces a bispecific antibody that binds CEA at two sites and HSG at one site, which can be applied to targeted prodrug radiotherapy.
The DNL method can further generate Fab2-scFv molecules (Figure 2-6), fusing scFv in the AD domain, and can be applied to a variety of specific antibodies including CD19, CD20, CD22, HLA-Dr, MUC5AC, Trop-2, and IGF-1R binds anti-CD3 antibodies to redirect T cells, showing the flexibility of this approach. In addition, DNL can be used to produce hexavalent, bispecific IgG-Fab4 fusion protein.
Other examples of non-immunoglobulin heterodimerization modules that may be used to generate bispecific antibodies include the barnase-barstar system, the aptamer/anchor tag module (based on the mutant RNase I fragment), and the SNARE module based on three proteins The interaction of synaptic fusion protein, small synaptophysin, and SNAP25 creates a binary system.
Albumin is a plasma protein that lacks antibody-like effector functions, but has a half-life similar to IgG molecules that can bind to FcRn for recycling. Two scFv and bispecific antibody molecule fusion albumin combine to produce bispecific fusion albumin-like, with albumin-like characteristics (Figure 2-6).
Therefore, two different scFvs are fused with human serum albumin (HSA), one is connected to the N-terminus and the other is connected to the C-terminus, which fully extends the half-life of scFvs. Similarly, fusing the bispecific scFv or scDb in tandem with albumin produces similar functions (Figure 2-6).
Using this method to produce scFv-HSA-scFv fusion protein, the scFv targeting HER2 and HER3 is fused into modified HAS (C34S, N503Q) to produce a fusion protein with good homogeneity, with short peptide linker AAS, AAAL is connected to the N end and C end of the HAS respectively.
Of course, various other proteins can be fused to produce bispecific fusion proteins, such as some toxins, cytokines, chemokines, growth factors, and bispecific antibodies to produce bispecific fusion proteins with effector functions.
Transplant other antigen binding sites to scFv
The simplest bispecific antibody consists of an antigen binding site scFv, which is modified to include a second binding site as a component of the scFv. Because immunoglobulin folding requires β-sheets to be connected, an attempt was made to graft complementarity determining regions (CDRs) to the bottom of scFv to generate bispecific molecules (χsFv).
Although this method has not been further used for the development of bispecific antibodies, related methods graft the antigen-binding site on the bottom of CH3. This principle can also be applied to other domains, such as grafting the antigen-binding site on CH1- The bottom of CL forms Fab.
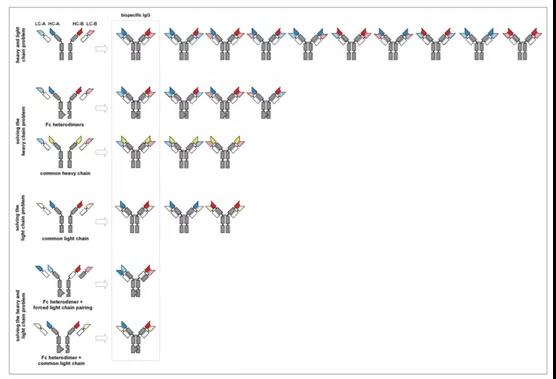
Figure 3. Combinations of different light and heavy chains and mismatch resolution strategies for light and heavy chains
Table 1. Fc heterodimerization design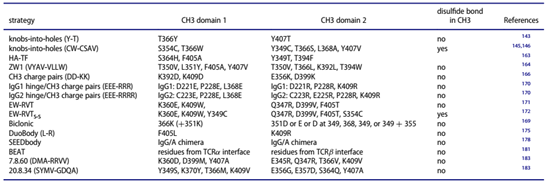
IgG-like asymmetric bispecific structure
All bispecific IgG molecules, bispecific antibodies are different from natural immunoglobulins. They have bivalent and asymmetric structures, and at least contain different Fv regions. The preparation of the heavy chain and the light chain is different from the origin, and they may also be different in the constant region of the heavy chain or light chain.
Asymmetric-like IgG contains heavy and light chains from two different antibodies
Fusion of two hybridoma cell lines produces a hybrid-hybridoma (quadroma), which produces a combination of two different antibody heavy and light chains. The bispecific antibody produced is composed of the heavy and light chains of the first antibody and the heavy and light chains of the second antibody (Figure 2-2).
Two antibody heavy chains and light chains can be of the same subtype and type, or of different subtypes and types. Two antibodies can also be derived from different species, and this method produces trifunctional antibodies. The fusion of mouse-derived hybridomas and rat hybridomas produces bispecific, asymmetrically structured hybrid IgG molecules (Figure 2-2).
The preferential pairing of heavy chain and light chain is described. It is important that the heterogeneous Fc part can be separated and purified by proteinA affinity chromatography, and the hybrid IgG molecule can be eluted at pH 5.8.
Furthermore, cell lines expressing different heavy and light chains can be achieved by means of genetic engineering. Genetic engineering methods allow different heavy and light chains to be composed of different human antibody types, and even allow the introduction of some mutations.
As described in the subsequent discussion section, it is allowed to introduce mutations in the heavy chain and light chain to facilitate the correct assembly of heterologous heavy chains and to promote the correct assembly of homologous heavy and light chains. At the same time, specific genetic engineering can also promote the correct assembly. Purification of assembled bispecific antibodies.
IgG-like asymmetric bispecific antibodies use Fc to solve the problem of heavy chain pairing
In the following chapters, we will focus on genetic engineering methods to solve the problem of heavy chain heterodimerization. The heterodimerized heavy chain can still bind different light chains to produce four types of antibodies, but there is only one bispecific molecule, one non-functional molecule, and two monoclonal antibodies.
The heavy chain heterodimerization method was used to reduce the number of 10 molecules formed by the random combination of four polypeptide chains to 4 (Figure 3). The pairing of the heavy chains relies on the last conserved domain. The CH3 domain in the natural IgG molecule mediates the pairing of two heavy chains, and the affinity between the two is KD~10 pM. With the help of the CH3 of the two heavy chains, the two identical heavy chains form a homodimer.
Further interaction occurs in the hinge region, and the two heavy chains are covalently connected by disulfide bonds. This is also the most important force with which we will refer to the assembly of heavy chain heterodimers. In the formation of homodimers of IgG1 heavy chains, the CH3 interface interaction of the two heavy chains involves 16 amino acid residues. The core amino acid residues include T366, L368, F405, Y407, and K409. Stability plays a pivotal role.
In the past 20 years, various strategies have been developed including spatial, electrostatic steering, or combinations of various designs, as well as the formation of interchain disulfide bonds. In short, the ultimate goal is to create complementary interfaces to overcome homology. The formation of dimers.
Inspired by the Knob-into-hole technology proposed by Crick et al. in the process of packaging two alpha-helix amino acids, Ridgway and his colleagues introduced this technology to the interacting CH3 interface, introducing a small molecular weight into the CH3 of a heavy chain. A Hole is generated from the amino acid of the opposite side, and a larger amino acid is introduced into the CH3 interface on the opposite side to generate a Knob. After trying different mutations, the mutation suitable for forming a heterodimer is finally determined. Do T366Y mutation in one CH3, and in the other One CH3 has Y407T mutation. This Knob-into-hole mutation was first used in IgG-like bispecific antibodies formed by anti-HER2 antibodies and anti-IGF-1R antibodies.
The Knob-into-hole strategy was further improved and perfected by phage display technology. These mutations were quickly used to produce IgG-like bispecific antibodies (Figure 2-7), and an attempt was made to introduce additional disulfide bond strengthening. The stability of heterodimers produced by Knob-into-hole technology. One of the excellent mutations is the S354C, T366W mutation in one CH3, and the Y349C, T366S, L368A, Y407V mutation in the other CH3 (Table 1).
This heterodimer formation strategy was subsequently used in the construction of anti-Mlp and anti-HER3 bispecific antibodies. Since the two antibodies contain the same light chain, they can express a common light chain. The heterodimer produces a functional bispecific antibody that can be purified by proteinA, and the antibody has an Fc-mediated ADCC effect.
Knob-into-hole technology has quickly been widely used, and has become a general bispecific antibody production strategy, and more theories and structures have been derived from it, including trivalent IgG-like antibodies, bispecific Fc , And bispecific CH3 fusion protein.
Another example of application is T cell redirection of bispecific antibodies. In order to avoid systemic activation of T cells due to bivalent CD3 antibodies, monovalent CD3 antibodies are used to bind to T cells with the aid of bispecific antibodies. This includes using scFv-Fc (KIH), that is, a scFv is attached to the N-terminus of each Fc. tandem-scFv-Fc(KIH) (BiTE-KIH) connects the tandem scFv to an Fc (Figure 2-9).
In this study, the CD3 antibody part can be fused to Knob-containing Fc (KIH) or Hole-containing Fc (KIHr). Interestingly, BiTE-KIHr outperforms BiTE-KIH in terms of expression titer. However, there is no difference in targeted T cell activation and tumor cell lysis. A similar method uses Fc-KIH to produce a bivalent bispecific scFv-Fc fusion protein that targets CD16 and HER2 and is used to recruit NK cells to the surrounding tumor cells.
Monovalent binding is necessary for some antibodies to bind to cell surface receptors, such as c-MET, in order to avoid activation caused by receptor coupling. Bispecific antibodies bind to cell surface receptors in a monovalent form. They are mainly used for dual-targeted binding and neutralization of different receptors. They are generally fused to the N-terminus of the Fc containing Hole by fusing the Fab arm to the C-terminus of the same Fc. The disulfide stabilized scFv co-expressed a non-fusion Fc chain containing Knob (Figure 2-9).
The VH domain is fused to the C-terminus of Fc (kih), and the VL domain is expressed alone or fused to the C-terminus of another Fc (kih) to produce a bispecific, trivalent IgG-Fv (mAb-Fv) fusion protein , Fv can be stabilized by interchain disulfide bonds (Figure 2-8). Fv is fused into IgG to form IgG-Fv. Protease cleavage sites can also be introduced during the fusion process. For example, furin or MMP cleavage sites can be introduced during the fusion of VL in Fv into Fc (Figure 2-8).
After digestion, a bispecific antibody with only the VH domain connected to the IgG at the Fc end was produced. Similarly, a scFv-Fc-Fv fusion protein was produced, containing two EGFR binding sites (scFv was fused into the N-terminus of Fc), the VH domain of an LPS antibody was fused into the Fc C-terminus containing Knob, and the VL domain Fusion into the C-terminus of Fc containing Hole (Figure 2-9). Bispecific technologies derived from KIH technology include TriMAbs.
In this technology, one or two disulfide bond-stabilized scFvs are fused into one or two chains in Fc (KIH) to form trispecific, trivalent or tetravalent antibodies (Figure 2-8). TriMAbs antibodies target EGFR, IGF-1R and/or cMET and HER3. In this method, the Fab is composed of a single-chain Fab, and the Fv domain accompanied by disulfide bond stabilization forms scFab-Fc(kih)-scFv2, scFab-Fc(kih)-scFv.
Recently, the knob-into-hole technology has been extended to other subtypes of IgG to produce IgG4 heterodimers, which lack Fc-mediated effector functions, such as the production of bispecific antibodies against IL-14 and IL-13. Using structure and sequence-based strategies, a variety of matching strategies for the CH3 interface have been developed. One mutation produces CH3 dimerization. This mutation is called the HA-TF mutation (S364H, F405A /Y349T, 394F).
Using this mutation, the formation rate of heterologous bispecific molecules in the form of mAb-Fv (IgG-Fv, mAb-Fv) was increased to ~83%. Using this method, a bivalent heterodimerization trivalent bispecific antibody targeting HER2 and monovalently targeting CD3 was developed. Further use of CH3 heterodimerization modules include monovalent and bivalent scFv-Fc fusion proteins.
Another rational design based on structure is to include a series of mutations. It is reported that these mutants make heterodimerized Fc have high thermal stability, and the formation of homodimers is not detected. The Fc(ZW1) mutation design includes the first chain mutations of T350V, L351Y, F405A, and Y407V, and the second chain mutations of T350V, T366L, K392L, and T394W, resulting in a bispecific IgG that contains a common light chain (Figure 2 -7, Table 1).
The ZW1 strategy mainly relies on the hydrophobic force. Another heterodimer formation strategy is to use the electrostatic steering strategy to avoid the homodimerization of the CH3 domain. After mutation, a positively charged CH3 and a band are generated. The negatively charged CH3 forms a dimer through electrostatic attraction. The charge interaction between K409 and D399 in the two chains was found in wild-type CH3. So the CH3 K409 of one chain was mutated to D, and the CH3 D399 of the other chain was mutated to K. This K409D/D399K mutation is very suitable to form a CH3 heterodimer.
The introduction of K392D in one chain of CH3 and the introduction of E356K mutation in the other chain will produce a specific heterodimer. Combining the above two mutations to produce K409D, K392D / D399K, E356K mutations is more conducive to the formation of heterodimerization This strategy has been used for the construction of bispecific fusion proteins in the form of CD3 and TARTK scFv-Fc (Figure 2-9). Recently, this method has also been used to generate targets for EGFR and HER2 or for sclerostin and DKK-1. Construction of IgG-like bispecific antibodies. This includes the introduction of charge pairing within the Fab arm to improve the correct pairing of the light chain.
Electrostatic steering is also used in Biclonics, the bispecific antibody uses common light chain technology and heterodimerization heavy chain strategy. In this bispecific antibody, one chain CH3 (366,366+351) is replaced by positively charged lysine K, and the second chain CH3 (349,351,355,368) is replaced by negatively charged glutamate E or The negatively charged aspartic acid D was substituted (Table 1). The bispecific antibody MCLA-128 against HER2 and HER3 produced by this technology has entered clinical phase 1/2.
The superior heavy chain heterodimerization strategy also includes the introduction of charged amino acids in the hinge regions of IgG1 and IgG2 (Figure 2-7). For example, for IgG1, D221E and P228E are used in the hinge region of the first chain, and D221R and P228 are used in the hinge region of the second chain (Table 1). For IgG2, the hinge region of the first chain is replaced by C223E, P228E, and the second chain is replaced by C223R, E225R, and P228R (Table 1).
Here, since E225R can have electrostatic attraction with E225 of the first chain, only two mutations are needed in the first hinge region of IgG2. Combining L368E and K409R to form mutations in the first chain C223E, P228E, L368E, and the second chain C223R, E225R, P228R, K409R, resulting in heterodimer assembly.
Using this method, an IgG-like asymmetric bispecific antibody against EGFRxHER2 was produced, and an IgG-like bispecific antibody against CD3Xcd20 was also produced. The half antibodies in the bispecific antibody were expressed separately, and the half antibodies were finally assembled into complete Of bispecific antibodies.
In another study, mutations were conducted in a direction that favored the formation of hydrophobic forces between the two chains. The hydrophobic interaction core amino acids L351, T366, L368, Y407 in the two chains are replaced by the symmetrical electrostatic attraction to the amino acids with greater or lesser hydrophobic interaction to form an asymmetrical hydrophobic interaction. Second, replace those amino acids with weakly charged interactions. , To form long-distance electrostatic interaction with long-chain charged amino acids.
As a result, the first chain CH3K360E, K409W mutations and the second chain CH3Q347R, D399V, F405T mutations, namely CH3 (EW-RVT) form (Table 1) were formed. With this strategy, the scFv-Fc heterodimerization bispecific antibody against VEGFR-2 and MET was formed, which has good biological functions (Figure 2-9).
The introduction of disulfide bonds (Y349C in the first chain and S354C in the second chain) into the above mutations will increase the formation of the heterodimer and correspondingly increase its thermal stability. In addition, the Fc binding library was screened by the yeast display system, and different Fc mutations were screened to increase the formation rate of heterodimers to 80%-90%.
Based on the observed dynamics of the natural Fab arm exchange of IgG4 antibodies, the two separate heavy chains are assembled into a complete IgG4. The exchange of Fab arms is mainly due to the combination of the core hinge region sequence with residues in the CH3 region.
This naturally occurring IgG4 Fab exchange introduces IgG1 and forms a stable IgG1 bispecific molecule (cFAE) by controlling the Fab exchange. The Fab exchange was controlled by screening CH3 domain mutations, introducing K409R mutations in the first antibody, and F405L mutations in the second antibody. These mutations allowed the two separately expressed antibodies to be mixed, in the presence of β-met (β-mercaptoethanol). ) Fab chain exchange occurs under the conditions of a reducing environment to form a bispecific antibody.
The anti-EGFRxCD20 bispecific IgG (DuoBody) produced by this method (Figure 2-7) produces >95% bispecific molecules.
The complementarity of the CH3 interface allows the assembly of heterodimerized Fc to form heterodimers via chain exchange engineered domains (SEED) (Table 1). Chain exchange engineered CH3 domain can select CH3 from human IgA to exchange CH3 with IgG (AG SEED CH3 and GA SEED CH3) to generate SEED bodies.
Since this molecular model study found that AG SEED CH3 reduced the interaction with FcRn, the interaction between IgG and FcRn would be restored by changing the amino acid residues linked to CH2-CH3. Pharmacokinetic studies have shown that the half-life of SEEDbodies is similar to that of IgG1.
Examples of SEEDbodies include bispecific Fab-scFv-Fc and scFv-Fc fusion proteins targeting two epitopes of EGFR bispecific antibodies (Figure 2-9). The bi-epitope antibody has similar binding power to the two monoclonal antibodies, but the biological activity is enhanced.
In addition, the structure similar to CH3 heterodimerization is that the α and β subunits of natural T cell receptors form heterodimers. This technology can be used as a TCR-based bispecific antibody construct (BEAT) (Table 1), can be applied to produce Fab/scFv-Fc fusion proteins, and can simultaneously solve the problem of light chain mismatches (Figure 2-9) ). This method has been used to generate bispecific antibodies against HER2 and CD3, which can redirect T cells.
Leaver-Fay and his colleagues applied multi-level design (MSD), which designs multiple protein levels and simultaneously generates a set of CH3 mutations. Two sets of mutation examples (7.8.60 and 20.8.34) (Table 1) are widely used to produce bispecific antibodies. The IgG-like bispecific antibodies produced by this method are composed of pertuzumab (anti-HER2), matuzumab (anti-EGFR) ), BHA10 (anti-LTbR), and MetMAb (anti-cMet) are derived. In combination with mutations in the orthogonal Fab interaction interface, up to 93% of bispecific antibody molecules are produced.
The heterodimerization assembly of the heavy chain can also be achieved by using a separate heterodimerization module, which is subsequently removed from the bispecific antibody. This method is applied to the heterodimerization of the leucine zipper structure. The basic operation is to fuse Acid.p1 (Ap1) and Base.p1 (Bp1) polypeptides at the C-terminus of the two heavy chains.
The LUZ-Y platform produces monovalent Fab-Fc fusion proteins, and can also produce bispecific IgG antibodies containing a common light chain, or produce scFab, which targets EGFR and HER3 (Figure 2-7). A protease cleavage site and a leucine zipper structure are introduced at the C-terminus of the Fc, and the lanine zipper can be removed by protease hydrolysis to produce bispecific IgG antibodies in a natural state.
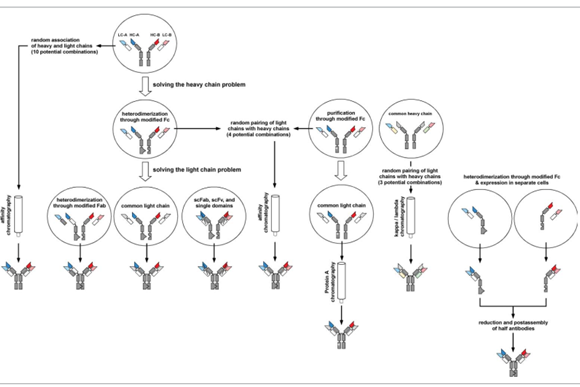
Figure 4. IgG-like bispecific antibody production strategy
Bispecific antibodies are produced by purification after assembly
The strategies described above are all based on modification to produce heterologous heavy chain pairing to complete the heavy chain assembly. The introduction of other mutations into the Fc region will facilitate the purification of heterodimerized antibodies. The post-assembly level is similar to the Triomabs strategy (Figure 4). The mutation introduced by Fc facilitates the purification of ProteinA by affinity chromatography.
For example, the substitutions derived from the corresponding amino acid positions of IgG3, H435R, Y436F, replace one chain of IgG1Fc with H435R and Y436F to produce Fc*. This mutation abolishes the binding to ProteinA (Figure 2-7). Therefore, the resulting Fc *- Fc* homodimers cannot bind to the proteinA affinity chromatography column, and the heterologous Fc Fc* bispecific antibodies have weakened affinity to proteinA.
However, ProteinA can also bind to the VH domain derived from VH3 family genes, which will interfere with the separation of FcFc and Fc Fc* dimers. This problem can be solved by the proteinA derivative Z-domain. This structure cannot be combined with the variable region and can separate the heterodimer from the homodimer.
Another method of using post-assembly purification strategy is to use a common heavy chain, different light chains, in this form of molecule, a given heavy chain variable region VH combines with a different light chain variable region VL to form a pair of different antigens The specificity. Therefore, only Fab arms exist in the asymmetric structure.
This strategy is used in k/λ bodies. This method requires the expression of three polypeptide chains, one heavy chain, and two light chains (1 kappa light chain, 1 lambda light chain) (Figure 2-2). The binding sites containing the common heavy chain VH can be screened through the shared VH domain library, or two antibodies containing the same heavy chain VH, or the VH of the existing antibody, and then combined with different light chains to screen for specific binding antigen Antibody.
It should be noted that one light chain needs to be Vκ and the other is Vλ. This strategy has been used in the construction of a variety of human antigen bispecific antibodies, which means that this technology can be widely used. The common heavy chain and common light chain are co-expressed with the same cell, and the subsequent bispecific antibody requires three-step chromatographic purification (1) The first step is to use IgG-ch1 capture selection column or ProteinA affinity chromatography, 2) κ chain selection column , 3) λFab affinity chromatography.
This method does not require genetic modification of the heavy and light chains to produce natural bispecific antibodies. However, the yield is much more mismatched than the heterodimeric antibody produced by mutation, and the mismatch rate is as high as 50%.
Using genetic engineering to solve the light chain mismatch problem in asymmetric antibodies
Using modification to force the heterodimerization of the heavy chain solves the problem of heavy chain mismatch. These strategies can also be used to solve the light chain problem. Therefore, using two different light chains will produce four different bindings, and only one molecule is a bispecific molecule. Many methods have been developed, combined with Fc modification (summarized in Table 1), to solve the problem of the correct pairing of homologous heavy and light chains (summarized in Table 2).
The first method used to solve the light chain problem is the common light chain. The basis of this method is that the same phenomenon as the light chain is often observed when screening antibodies against different antigens through a phage library, which reflects that the phage library of the light chain is very limited. Combined with the knob-into-hole technology of the heavy chain, IgG-like bispecific antibodies against HER3 and Mpl were generated. Various IgG-like bispecific antibodies containing a common light chain have emerged one after another, such as knob-into-hole-based modification, and other Fc modification methods for light chain.
Although it has not been used to generate bispecific antibodies, this is also an alternative strategy for common light chains. Surrobodies are based on Fab, using an alternative light chain, which is formed by fusing the λ5 domain with the VpreB domain. The binding library is used to screen antibodies against various antigens in the form of surrobody, such as the dual-targeting DR4 and DR5 inhibitory antibodies that are poorly developed using this method. Therefore, this method can also be used to generate bispecific antibodies.
Some of the previously described Fc modifications use scFv fusion Fc method to generate bispecific antibodies to circumvent the light chain problem. Based on the discovery that Fab can be expressed in the form of single-chain derivatives (scFab is connected at the C-terminus of the light chain to the N-terminus of the heavy chain, and Fab is expressed on a single chain), the full-length IgG molecule can be connected to the heavy chain through the light chain. To express the single chain, the linker uses 30-38 amino acids (G4S)6, and the disulfide bond site connecting CH1 and CL is deleted. An improved scFab platform includes a linker of 60 flexible amino acids, including disulfide bonds to stabilize the scFab.
(G4S)6 linker, the knob-into-hole mutation that binds to Fc can be used to produce Fab-Fc fusion protein. This structure can be further modified, through the C-terminal fusion of scFv, to form trispecific, trivalent and tetravalent molecules (Figure 2-7, 2-9), examples of this method are antibodies against EGFR, IGF-1R, cMet or HER3, using one Fab, and a tandem scFab arm, targeting two scFabs at different sites (Figure 2-8) .
scFab can also be combined with LUZ-Y Fc heterodimerization strategy (Figure 2-7). In addition, the protease cleavage site is introduced into the Fab connection, which means that the linker can be cut and connected from the correctly assembled IgG bispecific antibody by enzymatic digestion and hydrolysis. In addition, single-chain scFab can bind to unmodified Fabs to produce bispecific Fab-scFab-Fc fusion proteins, which bind to Fc (KIH) to form OAscFab-IgG format. The bispecific antibody against EGFR and IGF-1R produced in this format, and the bispecific antibody has a high expression level.
Table 2. Fab arm heterodimerization
CrossMab represents another strategy. In this technology, Zhonglian uses Knob-into-hole technology to generate CrossMabFab by swapping positions of heavy and light chains on a Fab, or only swapping the paired VH and VL positions in a Fab to form CrossMabVH-VL, or swapping one The positions of the constant regions of the heavy and light chains in the Fab are CrossMabCH1-CL (Figure 2-7). For example, the bispecific antibodies against VEGF and Ang2 produced by CrossMab technology, the crossMabCH1-CL form of the bi-antibody shows that the affinity remains unchanged, and the by-products are much less.
In subsequent studies, A2V antibody can reprogram macrophages. In a large number of extracranial tumor models, it significantly prolongs survival. The antibody RG7716 and RO6867461 produced by this technology has now entered the clinic. Recently, the CrossMabCH1-CL format was used to generate bispecific antibodies against the HIV membrane protein CD4/CCR5 to neutralize the virus.
Of course, some by-products can also be observed in CrossMabs at first, which are caused by unmodified light chain mismatches, which can be improved by introducing additional mutations later. CrossMabs has developed into a universal platform technology that can not only be used to produce bivalent, bispecific IgG molecules, but also trivalent and tetravalent bispecific IgG fusion proteins.
For example, fusion of Fab at the N-terminus of the heavy chain of Knob-into-hole, or fusion of two Fabs at the C-terminus of the two homodimerized heavy chains of CrossMab (Figure 2-1, 8). CrossMab technology can also be used for other Form antibodies, including bispecific, trivalent and tetravalent IgG-Fab fusion proteins, for example, a bispecific molecule is produced that binds CD3 with one binding site, and the other two binding sites bind tumor-associated antigens. Further produce tetravalent, tetraspecific 4 in1 antibody, by using Knob-into-holeFc region, and CrossMab technology to produce 2 in 1 Fab arms.
Another way to solve the light chain problem is to use genetic engineering to change the interface between the light chain and the heavy chain to generate orthogonal interactions, allowing the light chain to bind to the heavy chain of the hospital with high affinity (Table 2). This method mainly solves the VH-CL interaction in the variable region, and makes necessary modifications to the CH1-CL interaction. Try a variety of multi-level mutation designs for comparison and identification.
There is a set of mutations suitable for orthogonal Fabs. These modifications are used to generate a variety of IgG-like bispecific molecules such as EGFRxcMET, EGFRxHER2, Axl xcMET, EGFR xLTβR, and the use of two HER2 antibody trastuzumab and pertuzumab antigen binding sites to form bispecifics for two different epitopes of HER2 Antibody.
In some other cases, the heterodimerization of Fc is realized by electrostatic reversal. For example, the orthogonal Fab in a bispecific antibody uses pertuzumab (anti-HER2) and matuzumab (anti-EGFR). The light chain types of both antibodies are λ-type, and the heavy chain of the pertuzumab antibody is Q39K, R62E, H172A. , F174G substitution, D1R, Q38R, L135Y, S176W substitution (VRD1CRD2 modification) in the light chain, combined with the substitution of matuzumab antibody heavy chain Q39Y and light chain Q38R (VRD2 modification), resulting in up to 90% of the light chain assembly correctly.
A further strategy that directly affects the pairing between the light chain and the homologous heavy chain Fd involves the replacement of the heavy chain constant region CH1 and the light chain constant region CL in the Fab arm, respectively, with the Cα and Cβ domains of the TCR. This has been used to produce Fab-IgG-like molecules. The Fab arm is fused to the N-terminus of the heavy chain and connected with a (G4S)5 linker. IgG-Fab molecules can also be produced. The Fab arm is fused to the C-terminus of the heavy chain to ( G4S)4 linker link, such as bispecific antibodies produced by trastuzumab and pertuzumab (Figure 2-1).
However, due to the strong interaction between VH/VL at the antigen binding site of trastuzumab, only a small number of Fabs are correctly paired. The introduction of Y36F mutation in VL weakens the VH-VL interaction and improves it. In addition, the interaction between VL and VH is improved. The introduction of electrostatic interaction VL Q38D, VH Q39K can also achieve the purpose of weakening the VH-VL interaction (Table 2).
CH1-CL paired mutations further enrich the content of the trivalent Fab-IgG fusion protein. Based on the consideration of the 3D structure model and the energy perspective, two mutations were tested. Method one (CR3), the paired interaction of polar amino acids are mutated into neutral and salt bridges to form amino acids, the heavy chain CH1 has a T192E mutation, and the light chain CL has a N137K mutation. As a supplement, replace S114 with alanine in the light chain. Avoid steric conflicts with larger lysine side chains.
The second method is based on hydrophobicity-polarity interchange (MUT4). Two point mutations occur in both the heavy chain and the light chain. The heavy chain CH1 has L143Q and S188V mutations, and the light chain has V133T and S176V mutations (see Table 2). The method one (CR3) mutation in the in vitro test showed super activity, mainly due to the better stability of the bispecific antibody and the highest functional activity.
In the DuetMab method, the Fab arms are paired correctly, mainly by replacing the natural disulfide bond of CH1-CL to form a new replacement disulfide bond. Comparing numerous mutations, it was identified that the heavy chain CH1 F126C mutation combined with the light chain S121C, resulting in up to 98% bispecific molecules (Table 2).
Knob-into-hole technology combined with Fc will produce a variety of IgG-like bispecific antibodies. For example, bispecific antibodies against EGFR and HER2, or against CD4 and CD70, this structure produces bispecific antibody molecules with high purity and high activity (Figure 2-7).
After the production of half-antibody assembly to solve the light chain problem
As mentioned above, the problem of correct assembly of different heavy and light chains is solved through genetic engineering. All assembly takes place in the same cell. However, it is also possible to express the two half-antibody molecules (a heavy chain and a homologous light chain) that constitute a bispecific antibody separately and then assemble to form a bispecific molecule (Figures 3 and 4).
In this way, the introduction of mutation modification in the heavy chain Fc is sufficient to develop in the direction that is conducive to the formation of heterodimers, or the assembly after proteinA or proteinG affinity chromatography is completely conducive to the formation of heterodimers. This concept has been demonstrated in the IgG-like bispecific antibody model that contains knob-into-hole and no hinge region in Fc. The two half-antibodies are expressed in E. coli, and then purified by proteinA and other chromatography, and then the two half-antibodies are renatured to produce bispecific IgG molecules.
A further improvement of this method is to co-culture two E. coli expressing half antibodies. By lysing the bacteria, the two half antibodies are released and assembled into a complete bispecific antibody. This method has been validated in the formation of IgG-like bispecific antibodies from anti-EGFR and anti-MET antibodies. The two E. coli clones that produce the corresponding antibodies need to be adjusted to the optimal ratio.
Therefore, after trying, the ratio of anti-MET:anti-EGFR is 60:40. Finally, the half-antibody was purified by ProteinA affinity chromatography and hydrophobic interaction chromatography. An important point of the co-culture method is to eliminate the redox step. This method has been used in anti-IL13xIL4, or anti-HER2xCD3 bispecific antibodies. This neutralizing antibody does not require ADCC and other Fc-mediated effector functions.
The post-expression assembly strategy can be further used in the hinge region, as well as the process of assembly of charge-paired mutants of CH3 domains (IgG1 (EEE-RRR) and IgG2 (EEE -RRRR) into bispecific antibodies (Table 1, Figure 2) -7).
The two antibodies can be expressed separately in HEK293 cells and purified by proteinA affinity chromatography. The purified antibody is then placed in a mild reducing environment to produce half-antibodies, and then the two half-antibodies are mixed in an equimolar manner and then gently reduced. Under conditions, incubate overnight at 37°C.
The mammalian expression system can be used to produce antibodies with glycosylation modification without changing the Fc effector function. This method is also suitable for producing Knob-into-hole IgG-like bispecific antibodies.
Asymmetric Fc and CH3 fusion protein
The above-mentioned Fc modification can be used to produce bispecific Fc or CH3 fusion proteins, and many examples have been reported. Various binding modules, scFv, Fab, scFab units, etc., have been used in the construction of bispecific antibodies. Fusion of two scFv targeting different targets on each Fc produces a bivalent bispecific scFv-Fc fusion protein with the characteristics of IgG. This strategy can also use the aforementioned Knob-into-hole Fc region, CH3 domain charge-paired heterodimerization Fc, and EW-RVT modified Fc (Figure 2-9).
In addition, one Fab can bind to one scFv, for example, one scFv is fused to the N-terminus of each heterodimerized Fc to produce a Fab-scFv-Fc fusion protein (Figure 2-9). This method has been used for BEAT Fc modification.
A variant of this method is to use CH3 domains to form heterodimers, such as bispecific scFv CH3 fusion proteins (Figure 2-9), such as a bivalent bispecific fusion protein produced by this method Minibody, the fusion protein fused an anti-HER2 scFv to the N-terminus of a CH3 domain, fused an anti-CD16 scFv to the N-terminus of another CH3, and introduced a disulfide bond at the C-terminus of the two CH3s to further stabilize CH3 dimerization structure (Figure 2-9).
Further heterodimerization of the Fc region can be used to fuse two binding sites into one Fc, without fusing the other Fc, and then co-express the two Fc (Figure 2-15). For example, a bispecific Fab-Fc-scFv fusion protein, fused anti-Met Fab into the Fc N-terminus containing hole mutations, and fused anti-digoxigenin disulfide bond-stabilized scFv into the Fc C-terminus containing hole mutations, and The other unfused Fc containing knob was co-expressed to form a heterodimer.
In one study, a bispecific tandem scFv was fused to the N-terminus of Fc to produce a bispecific, bivalent fusion protein. In another study, a DART-Fc fusion protein was produced. One chain of the DART molecule was fused into the Fc N-terminus with a knob mutation, and then it was combined with an unfused Fc with a hole and the other chain of DART. Co-expression.
Another method is to use the knob-into-hole dimerized Fc to fuse one DART chain into the Fc with the knob mutation, and then fuse the other DART chain into the Fc with the hole. DART’s disulfide bond covalent linkage replaces the hinge region linkage. The DART-Fc fusion protein is being developed for the development of a CD3 bispecific fusion protein for T cell redirection.
In order to avoid the functional effects caused by Fc, an effect-deficient Fc was introduced. These methods for forming bispecific, bivalent antibody properties can be combined with some strategies to extend half-life.
Bispecific antibody with symmetric structure
IgGS episome: scFv fusion protein
Fusion of additional binding sites to the heavy or light chain is the most direct way to solve the problem of random pairing between heavy and light chains. This requires the additional binding site to be expressed in the form of a polypeptide chain. Moreover, this polypeptide link is not allowed to interact with the heavy and light chains of the host antibody. scFv is the most suitable fusion partner, not single domain antibody and scaffold protein. This IgG-based episome has a symmetrical structure and four valences to bind to the antigen. It contains two binding valences for each antigen and can be expressed by four peptides in conjunction (Figure 1).
This method was first reported in 1997. Coloma and Morrison1 fused anti-dansyl scFv into the C-terminus of the heavy chain CH3 of anti-dextran, or the C-terminus of the hinge region. A short linker G3S is connected between the C-terminus of the heavy chain and the N-terminus of the VH domain of scFv. Co-expression of the light chain of anti-dextran produces an IgG-HC-scFv (CH3-scFv) or F(ab’)2-scFv2 (Hinge-scFv) fusion protein. The structure contains four binding sites (Figure 2- 10, 16).
The bispecific fusion protein IgG-scFv has a molecular weight of 200KDa, and F(ab’)2-scFv2 has a molecular weight of 150KDa. The bispecific antibody binds to two antigens and occasionally encounters a decrease in scFv affinity.
The further derivation of this method is to fuse an scFv at the N-terminus of the heavy chain (Figure 2-1) or light chain (Figure 2-11) of the antibody to produce scFv-HC-IgG, scFv-LC-IgG, or in the heavy chain A scFv is fused to the N-terminus of the light chain to produce scFv-HC/LC-IgG (Figure 2-12). The introduction of disulfide bonds in the Fv region will improve molecular stability and reduce aggregation. The fusion protein maintains a high level of expression, is thermally stable, and is resistant to protease cleavage.
They also retain the effector function of Fc and the half-life similar to IgG. Fusion of an scFv to the C-terminus of an IgG light chain can also produce a functional bispecific antibody IgG-LC-scFv. Many of these antibodies maintain stability and have the same characteristics as normal IgG. In some antibodies, disulfide bonds are distributed between the heavy and light chains. Therefore, both IgG heavy and light chain ends can be used to generate episomes, bispecific molecules.
Further studies have shown that tandem scFv fused to the N-terminus or C-terminus of the heavy or light chain can produce trispecific antibodies. Further imagination includes the construction of multivalent molecules, including tri- or multispecific antibodies, which can be fused with the same or different scFv molecules in the heavy and light chains of the antibody. Although it may affect the binding of the binding site to the antigen, it has been optimized and Screening can achieve the desired goal.
Fusion of a scFv to the CH1 of the heavy chain and another scFv to the CL end of the light chain can produce a tetravalent, tetraspecific antibody scFv4-Ig, containing four scFv molecules at both ends of the constant region (Figure 2-12 ), this method was originally used to produce bispecific antibodies targeting two different epitopes of VEGF, and later it was also used to dual target anti-EGFR x anti-IGF1-R bispecific antibodies to tumor cells. Sexual antibody construction.
The same format can also be used to construct EGFR-specific scFv combined with anti-CD3 scFv bispecific antibodies to redirect T cells to tumor cells. Comparing the bispecific antibody of this structure with the equivalent scDb-Fc fusion protein, it showed the highest cytotoxicity. However, due to its high molecular weight of 200KDa, the yield of recombinant protein is very low, so scDb-Fc fusion protein has more advantages in general.
A key issue to consider for IgG scFv is the linker that connects scFv to IgG. The linker must be stable and ideally flexible, and cannot interact with scFv and antigen IgG binding domains. Fusion of scFv into the C-terminus of IgG light or heavy chain cannot affect the binding of IgG to antigen.
However, some scFv attached to the N-terminus will block the binding site of IgG and antigen. The typical IgG-scFv fusion protein linker is 2-3 repeats of G4S. Other linkers include some hydrophilic helical linkers, such as SNS (EEAKK) 3SNS has been used to fuse scFv into the C-terminal link of the heavy chain. Short linkers may have the ability to maintain antigen binding. The last three-residue linker GSS has been successfully used in the construction of IgG-HC-scFv targeting HER2 X HER3.
Furthermore, the natural stability of scFv may be affected by the process. Therefore, in order to optimize the stability of scFv, a longer linker (20 amino acid residues) was used to connect the VH and VL domains, and suitable sites were selected to introduce disulfide bonds to strengthen the interaction between the two.
Combine long linkers and introduce disulfide bonds between VH44 and VL100 to stabilize scFv. In the example of anti-LTbR scFv, using this strategy to improve its thermal stability by 13℃, the anti-TRAILR2 x anti-LTbR IgG-scFv bispecific antibody and scFv-IgG fusion protein produced by this strategy are ideal drugs. Use development value.
Disulfide bonds can also be used to improve the stability of bispecific IgG-scFv and IgG-HC-cFv. In order to improve the stability of anti-digoxigenin-specific scFv, a disulfide bond is formed between VH Cys44 and VL Cys100. It is fused to different specific antibodies, including HER2, IG1-R, CD22, and LeY heavy chain C-terminal or hinge region.
In the above structure, the scFv is connected to the C-terminus of the heavy chain by two repeating G4S linkers, and all antibodies maintain affinity and specificity. All bispecific antibodies are very stable, and no protein aggregation is seen. This strategy then uses other antibodies, including cMet, HER1, HER2, or HER3.
The influence of the linker length has also been studied in the IgG-scFv fusion protein of the epitope bispecific antibody at two different sites of the HIV receptor CCR5. 3-6 repeated G4S linkers have also been studied in non-disulfide bond-stabilized scFvs.
A linker of up to 30 amino acid residues is used to link the scFv, and its stability is similar to that of the parent antibody, but a linker of 15 amino acids is used in the scFv stabilized by the introduction of disulfide bonds.
Screening for more stable scFv with the help of phage library can partially solve the limitation of scFv stability. Early research on this aspect can be seen in the research of anti-IL17A x anti-IL23 bispecific IgG-scFv fusion protein.
The final antibody screening is based on the detection of the two connection methods of scFv, VH-VL and VL-VH, SEC-MALS to detect the stability of the components, and scanning calorimetry to evaluate the thermal stability of the monomers. Comprehensive screening of the best molecules . Similarly, the stability-optimized scFv has been used to produce bispecific antibody construction targeting different epitopes of IGF-1R, and the scFv is fused to the N-terminus or C-terminus of IgG.
IgG scFv fusion protein can exert Fc-mediated effector functions, including phagocytosis, ADCC, and complement fixation. The effector function depends on different antibody subtypes and structural forms. For example, in some applications, antibodies that dually target and neutralize cell receptors will not destroy target cells. At this time, IgGs carrying defective Fc are more advantageous.
Therefore, a complete Fc region with no effector function can be replaced by a deglycosylated IgG4.P/IgG1 constant region chimera. This structure has been used to construct an IgG-HC-scFv-like fusion protein to improve stability The optimized anti-IGF-1R scFv is fused to anti-EGFR IgG. Other Fc with no effector function include some Fc mutations, or chimeras derived from IgG2 and IgG4.
In the past few years, a variety of bispecific IgG-scFv fusion proteins have been constructed for tumor therapy. They have applications in dual-targeting, antibody drug delivery across the blood-brain barrier, and pre-targeted radioimmunotherapy.
IgGs episome: fusion antibody domain and scaffold protein
As an alternative to scFv, single domain antibodies have been used to generate episomal molecules specific for IgGs. For example, the single variable region domain of anti-PDGFRa is connected to the N-terminus of the anti-VEGFR-2 IgG light chain through a 5-amino acid (ASTKG) linker, maintaining the antigen binding activity and neutralization effect of the two antibodies (Figure 2-11), the method was subsequently expanded, and a single domain was fused to the C-terminus of the heavy chain (Figure 2-10).
With the development of various scaffold proteins as alternatives to antibodies, there are more diverse opportunities to fuse scaffold proteins to the heavy or light chain of IgG to produce bispecific IgG episomal molecules. An example is Fynomers, a globulin with a molecular weight of only 7KDa, which is derived from the SH3 domain of human Fyn kinase. This protein lacks disulfide bonds, but is very stable, with a melting temperature of 70°C.
The protein is fused to the N-terminus or C-terminus of the heavy or light chain of the anti-HER2 antibody pertuzumab to produce bispecific molecules (FynomAbs), which have the ability to neutralize HER2-mediated tumor cell proliferation. Based on these findings, another FynomAb, COVA322, is fused to the anti-tumor necrosis factor TNF drug Humira with anti-IL-17A Fynomer, COVA322 is currently used in the phase 1b/2a clinical trial of Yin Xiao disease.
Related scaffolding protein uses include specific polypeptides, and they can also be used as fusion with IgG to produce bispecific molecules. This strategy has been applied to Zybody technology (Figure 2-12). For example, an Ang2 specific polypeptide is connected to the C-terminus of the heavy chain of anti-TNF IgG (adalimumab) via a 6-amino acid residue Gly-Ser linker.
Zybodies produces up to 5 specific binding sites, one recognition site comes from IgG, and the remaining four binding sites come from fused recognition domain modules, including linkers, disulfide bond-containing polypeptides, Affibodies and Knottins Fusion at the N-terminus and C-terminus of the heavy chain and light chain.
Episomal bispecific antibody molecules can be further formed by fusion of IgG to a receptor domain that can bind to a ligand. Although different from classic bispecific antibodies, this molecule still exhibits two different specificities.
IgGs episome: fused Fab arm
Fusion of Fab arms on IgG, for example, tandem Fab fusion on Fc is used to produce a tetravalent (Fab)2-Fc fusion protein, thus forming an Fd-extended heavy chain, which is expressed with light chain to produce Tandemabs. This format can be used to produce tetravalent bispecific (Fab) 2-Fc fusion proteins. The production of bispecific molecules here also faces the problem of light chains, because there are 8 possible combinations, but only one is bispecific.
Brunker and his colleagues used CrossMab technology to generate bispecific IgG-Fab molecules, and ligated the second Fab to the IgG molecule with (G4S)4 (Figure 2-7). In this method, the fused Fab is connected in the manner of VH-CL and VL-CH1. This method has been used to produce trivalent bispecific antibodies that can target fibroblast activation protein (FAP) for targeting Activated tumor stromal fibroblasts, another antigen binding site is DR5 (TRAIL receptor 2), to induce apoptosis through death receptors (Figure 2-10).
There is a difference in the order of VHCL and VLCH1. In the form of VLCH1, the VL domain is fused to the C-terminus of HC, which reduces the binding to FAP compared with the parent antibody, while the binding activity of the antibody in the form of VHCL (VH fused to the C-terminus of HC) remains unchanged. Combined with the knob-into-holeFc region, this method can also produce a trivalent, bispecific IgG-Fab fusion protein.
As described above, orthogonal Fab can be used to generate trivalent, bispecific Fab-IgG fusion proteins (Figure 2-10). Here, the outer Fab arm is connected to the heavy chain VH through Fd, and the inner Fab is connected through (G4S)5. This method has been used to produce bispecific Fab-IgG, such as anti-HER2 Fab-IgG, anti-HER2 can be derived from For pertuzumab and trastuzumab. Compared with scFv-IgG fusion protein, Fab-IgG fusion protein has the same specificity, but has superior biophysical properties and unique biological activity.
The Fab-IgG fusion protein can also introduce charged residue mutations or hydrophobic-polarity exchange mutations into CH1-CL. In this study, the Fd fragment of the first antibody was fused with a hinge region (wild type or with two cysteines mutated to serine), and then connected to the CH1 heavy chain containing CR3 or MUT4 mutations through the STPPTPSPSGG linker.
The homologous light chain of the inner Fab binding site contains corresponding mutations. Therefore, the heavy chain in the molecule contains a wild-type hinge region covalently connected by a disulfide bond formed by the additional hinge region between two Fd (Figure 2-10). The bispecific Fab-IgG fusion protein is functionally trivalent, with the outer Fab arm targeting HLA-DR and the inner Fab targeting CD5.
Good stability, limited protein aggregation, in vitro experiments show that CD5 x HLA-DR (CR3) molecules have good biological activity. Molecules that do not contain cysteine in the hinge-containing region of the internal Fab show a certain degree of reduced binding capacity, and in some aspects, such as complement activation, the effector function is weakened.
As described above, Fabs containing TCR Cα and Cβ domains should be connected to the N-terminus or C-terminus of the heavy chain of the second Fab arm with (G4S)4, as described above, derived from the bispecific formation of trastuzumab and pertuzumab Sex antibody.
The introduction of additional mutations in the VL domain (Y36F) in one Fab arm to weaken the VH-VL interaction, combined with the charge interaction (VL Q38D, VH Q39K) caused by the mutation between VL and VH, further promotes the homologous Fab arm Pair correctly.
The DNL technology described above is used to generate a hexavalent, bispecific IgG-Fab4 molecule (Figure 2-19), which is prepared by mixing DDD2 Fab fusion protein, AD2 IgG fusion protein under redox conditions and purified by proteinA. Therefore, this method produces 2+4 molecules with two binding sites for IgG to recognize the first antigen.
The other four antigen binding sites from the Fab arm are for the second antigen. DDD2 can be fused to the N-terminus or C-terminus of the FabFc chain. A further AD2 domain is fused to the C-terminus of the heavy chain or light chain. In HexAbs, AD2 is fused to the C-terminus of heavy chain CH3, the complement effect is relatively weakened, and other Fc-mediated functions such as ADCC are still retained. Some hexavalent antibodies (bsHexAbs), such as hexavalent antibodies against CD20 and CD22, exhibit biological functions that cannot be achieved by mixing the two parent antibodies, resulting in improvements in induction of apoptosis and inhibition of proliferation.
Although its molecular weight has reached ~350KDa, compared with the parent antibody, in mice, although the tissue uptake is similar, the half-life is reduced by 2-3 times. This may be due to the degradation of bsHexAbs in the cell.
IgGs episome: fusion of additional heavy and light chain variable domain domains
The second specific variable region domains VH and VL are fused to the heavy and light chains of IgG, respectively, to produce the so-called dual variable region antibody DVD-Ig (Figure 2-12). The original DVD-Ig was used to construct bispecific antibodies targeting IL-12 and IL-18. This format has been used for the construction of bispecific antibodies of a variety of antibodies, including IL-1a and IL-1b, HIV gp41 and Antibodies to two different epitopes of gp120, CD20 and CD47, EGFR and HER3, CD20 and HLA-DR, and HER2, CEA and DOTA. Analyzed a series of DVD-Igs, targeting TNF and another alternative therapeutic target. The positions of the two binding sites are interchanged (internal and external interchange) for the different linkers on the heavy and light chains. The link length, ranging from 5-13 amino acids, has been studied one by one on these factors affecting antibody function.
The results show that when the binding site for TNF is located inside, the affinity is lower, which may be the cause of steric hindrance, resulting in a decrease in the binding rate. When two variable regions are on one chain, the linker also affects binding. In another gp41 and gp120-specific DVD-Igs study, the results showed that the internal binding site requires a linker of at least 15 amino acids in length, and the choice of spiral linker is more effective for DVD-Igs than flexible linkers.
Using single particle electron microscopy to study DVD-Ig, it is found that its outer binding site has a high degree of fluidity and can be folded out of the plane to bind antigen to the inner binding site, while DVD-Ig binds IL-12 and IL- The 3D structure of 18 further confirms this.
Further research on DVD-Ig technology is to introduce protease cleavage sites, such as metalloproteases cleavage sites, between the external and internal VL. These activatable DVD-Igs can target external binding sites, such as the cell surface, and can bind to another antigen by restriction enzyme digestion. This method is used for aDVD-Ig targeting ICAM and TNF to enhance the specificity of the inflammation site and reduce the damage to the inflamed tissue caused by off-target.
The crossover dual variable domain Ig-like protein (CODV-Ig) represents another form: two VH and two VL domains are connected to each other through cross-pairing to form a VH-VL domain, and their arrangement order can be changed ( From N end to C end), in the order of VHA-VHB and VLB-VLA or VHB-VHA and VLA-VLB (Figure 2-12).
Therefore, whether it is a light chain or a reconnection, the order of the variable region can be changed, and the other chain uses the order of the first chain as a template, so a total of four permutation structures will be produced. Since the cyclic self-contained structure is only generated in CODV-Igs, the CODV-Ig format is different from the DVD-Ig format. The CODV-Ig format has been used to construct bispecific antibodies against IL-4 and IL-13, TNF and IL-12/23.
Linkers (L1 to L4) have different lengths and include all-glycine linkers. They are usually used to connect two variable regions and connect the internal variable region to the first domain of the constant region. Although the length and sequence of the linker affect the yield and protein aggregation tendency, the study found that almost all the analyzed linkers can achieve the correct pairing of Fv and Fc. Through the three-dimensional structure of anti-IL-4 x anti-IL13 CODV-Fab, the tight assembly of its binding site was found and the complex formed with the antigen.
Modified IgG molecule
Symmetrical bispecific IgG molecules, such as modified IgGs, can recognize both by modifying the VH and VL domains (2-in-1,4-in-1 antibody) or transplanting additional binding sites on the C-terminus of Fc. A different antigen (mAb2).
The engineered antibody variable region domain Fv targets multiple antigenic sites to produce structurally invariant bispecific antibodies. Because the Fv region consists of two variable domain domains (VH, VL), each group of VH and VL recognizes one antigen, and the two groups of VH and VL can recognize different antigens, so bivalent bispecific antibodies can be produced ( 2-in-1 antibody) (Figure 2-18).
A bispecific IgG targeting HER2 and VEGF, derived from bispecific Fabs, the light chain of the antibody is from Herceptin (trastuzumab), which mimics amino acids by randomly selecting a subset of VL CDR sites exposed to solvent Natural diversity in composition and length. Through further screening and affinity maturation, a variety of IgGs can be produced that can bind HER2 and VEGF at (nM) concentrations.
The crystal structure shows that despite the extensive overlap, Fab binds well to both HER2 and VEGF. In vitro experiments show that the bispecific antibody with 2-in-1 structure has a good neutralization effect on the two antigens. Further research has shown that the structural plasticity of the antigen binding site is enhanced without increasing the entropy cost.
This method was subsequently applied to optimize the HER1 x HER3, IL-4 x IL-5 2-in-1 bispecific antibody. The HER1 x HER3 2-in-1 bispecific antibody increases its valence to the receptor and is expected to overcome the effects of HER2 inhibition and radiotolerance. This antibody MEHD7945A has entered clinical phase 1 and phase 2 trials. Studies have shown that the drug has very good efficacy and has obvious anti-squamous cell carcinoma and head and neck cancer effects. In the 2-in-1 format, the 6 complementarity determining regions (CDRs) are modified to recognize the two, so that overlapping antibody epitopes are generated.
Another way is to use DutaMab and DutaFab formats (Figure 2-18). In this model, the antibody binding site has 6 CDRs, defining 3 antigen binding sites that make up the Fv. H1, H3, and L2 of CDRs bind one antigen, and L1, L3, and H2 of CDRs bind another antigen. The two groups of Fv composed of 3 CDRs have different orientations. Therefore, as long as the two antigens do not interfere with each other in size and morphology, one Fab arm of DutaFabs and DutaMabs can simultaneously bind two different antigens (or recognize two different pairs of antigens in one IgG).
In addition to the CDR loop loop in the variable region domain, other loop structures exposed on the surface can also be used to create artificial binding sites. This method has been successfully used in the IgG1 Fc region. Initially, two loop sequences were randomly selected at the c-terminus of the CH3 region for antigen binding. As shown by HER2, the Fc fragment (Fcab) produced has nano-level affinity. More importantly, the randomized fragment did not affect the binding to ProteinA and CD64. The stability of the Fcab fragment was further improved by engineering the introduction of intrachain disulfide bonds and direct yeast display evolution.
The three loop loops (AB, CD, EF) of the CH3 domain can be selected for randomization. The second inhibitory Fab arm is established by Fcab to produce a bispecific IgG molecule (mAb2). This structure maintains all the characteristics of IgG, including half-life and production preparation (Figure 2-18).
Bispecific antibodies based on symmetric Fc and CH3
In natural antibodies, the main function of Fc is to form a homodimerization module, and it also mediates corresponding effector functions, such as ADCC, phagocytosis, and complement fixation. Using the Fc region, it usually further includes a hinge region to form a disulfide bond covalently connected. The bispecific antibody can be fused with the other half of the bispecific module at the N-terminus of the antigen-binding site, or the antigen-binding site at the N-terminus and C-terminus. , Such as scFv, produces tetravalent bispecific antibodies.
This method was applied to a proof-of-concept study of two scFvs targeting TNF and fluorescein isothiocyanate (FITC). The first scFv (anti-TNF) is fused to the Fc of IgG1 with a modified hinge region, and the second scFv (anti-FITC) is connected to the C-terminus of the Fc. The connector was tested, including (G4S)3AAA connector, A4T connector (T(A4T)3AAA), and a CL connector (EGKSSGASGESKVDAAA) (Figure 2-13).
All three linkers can form dual targeting molecules, similar to the stability of scFv-Fc-scFv fusion proteins. The A4T linker was then used to generate scFv-Fc-scFv fusion proteins for IT1 and CXCL13. The protein shows monoclonal antibody-like properties such as yield, stability and viscosity. This method is further used to generate trivalent bispecific antibodies against LPS and EGFR, which use disulfide bond-stabilized scFv linked to Fc via a G4S linker.
In another method, scFv molecules are arranged in tandem, and each scFv forms a separate folding and binding unit, which is fused to Fc. For example, tetravalent, tandem scFv targeting different epitopes of CD2 produces bispecific taFv-Fc fusion protein, which can induce mitotic proliferation of T cells in the resting phase.
A 24-amino acid helical linker is used to connect the two scFv parts. The taFv-Fc format can also be used to produce tetravalent bispecific Fc fusion proteins. For example, the tetravalent bispecific Fc fusion protein targeting C5 and cotinine is used in immunoblotting and immunoprecipitation experiments. Two scFvs in this structure are linked by a (G3S)3 linker, and the tandem scFv is fused to the hinge region of IgG1 Fc. The scFv bound to cotinine serves as a recognition module for conjugated cotinine, for example, horseradish peroxidase (HRP).
In another study, the taFv-Fc fusion protein targeting HER2 and cotinine can be used for ELISA and radioimmunoassay. The histidine dipeptide coupled with HRP-cotinine or 125I-cotinine is used for single-photon emission CT imaging of her2 positive tumors. Other taFv-Fc fusion proteins include TAG-72 and MUC1 or EGFR and HER2 for dual-targeting tumor-associated antigens.
Other uses include T cell redirection (for example, against GD2 and CD3), and target-dependent aggregation and activation of death receptors, such as the taFv-Fc fusion protein that recognizes DR5 and melanoma-related chondroitin sulfate proteoglycans.
Instead of fusing two scFv to the N-terminus of Fc, one Fab arm can also be fused to scFv-Fc (Figure 2-13) to form a Fab-scFv-Fc fusion protein. The expression of Fab-scFv-Fc fusion requires four polypeptide chains, one for editing the FdscFv-Fc part and one for editing the light chain.
This form has been used to produce tetravalent bispecific antibodies (BiS4aPa). The target is two different extracellular polysaccharide antigens (PcrV and Psl) from Pseudomonas, or to treat Pseudomonas infection.
The antibody scFv is inserted between C220 and D221 in the upstream hinge region, and is connected with a 10-amino acid linker G4SG4S. The antibody drug (MEDI3902) has entered clinical phase 2 (EVADE, NCT02696902), and its main purpose is to prevent nosocomial pneumonia caused by mechanical ventilation equipment.
The bispecific diabody format is used to generate Fc fusion proteins by fusing one of the diabody chains with a covalently linked CH3 domain or Fc chain (Figure 2-13, 14). This will produce so-called Di-Diabodies, the structure is tetravalent, bispecific molecules constitute two chains, a VLB-VHA CH3 or VLB-VHA-Fc chain, co-expressed with the second chain VLA-VHB.
This method has been used to produce Di-Diabody targeting EGFR and IGF-1R. Although Di-Diabody shows dual receptor neutralizing activity in binding capacity, in vitro experiments have shown that the binding capacity has been weakened, which may be mainly due to Because the two chains dissociate in the cycle.
The remarkable stability of scDb is due to the covalent linkage of the four variable regions of the bispecific diabody. Fusion of a scDb to Fc produces a trivalent bispecific molecule that is similar to IgG in molecular weight and pharmacokinetic properties.
The Fcab format mentioned above can be used to produce scFv-Fcab fusion proteins, which are encoded by one chain (Figure 2-13). There are two antigen binding sites at the C-terminus of Fc, and the N-terminus of Fc fuses these two additional antigen-binding sites in the form of scFv. This form has been used to produce HER2 and CD3 bispecific antibodies, scFv binds to CD3, and the tumor cell killing effect of T cell redirection has been seen in in vitro experiments and animal models.
Bispecific antibodies derived from homodimerization of immunoglobulin derivative domains
A variety of immunoglobulin domains form homodimers, such as CH3 in IgG, IgA, IgD, IgM, and CH4 in IgE. In addition, CH2 in IgM and IgE functions as a hinge region, connecting the Fab arms with CH3 and CH4. Unlike IgG, IgA, and IgD, the CH2 in IgM and IgE is covalently linked through 1-2 disulfide bonds, and there are additional N-linked glycosylation sites. These domains can be used for anchoring to produce tetravalent bispecific molecules (Figure 2-14, 15). For example, a scFv or a diabody can be fused to the N-terminus and C-terminus of IgM, IgE CH2, or a single diabody can be fused to the N-terminus of CH2. Chain diabody (scDb-CH3).
Table 3. Clinical research progress of bispecific antibodies
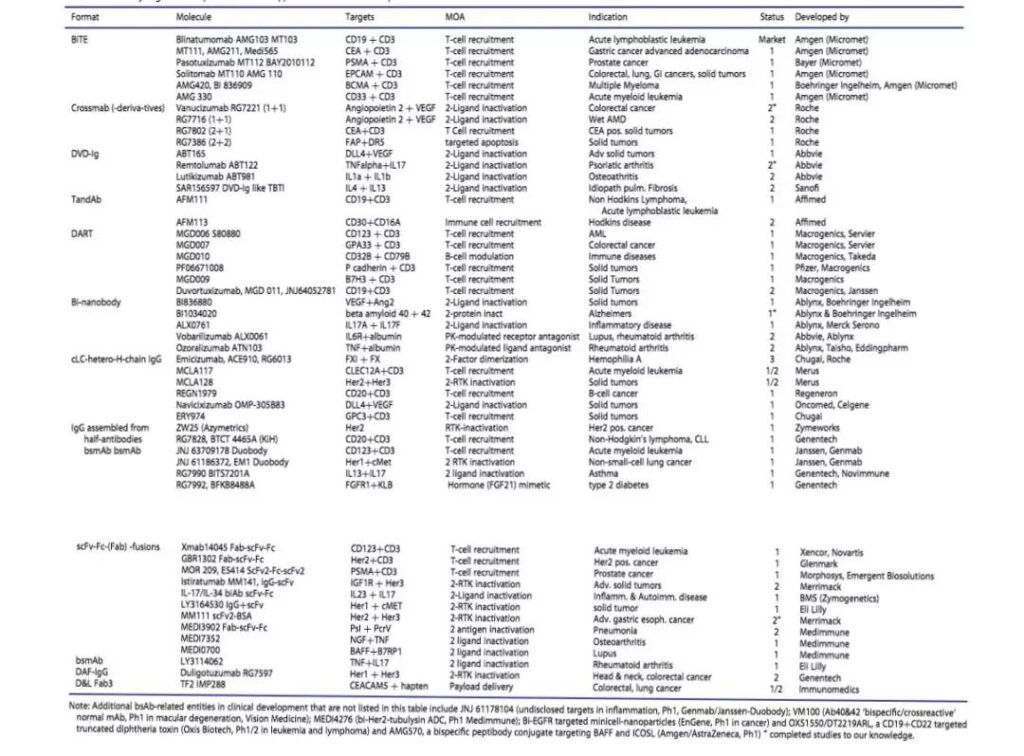
Prospects for the Industrialization of Bispecific Antibodies
The bispecific antibody format is shown in Figure 2, which illustrates more than 100 different formats, which are the construction forms of dual antibodies and their derivatives that we currently know. This article aims to include all conceptually different experimentally verified formats described in the literature as of September 2016, but we have to admit that we have overlooked some structural forms. Antibodies are composed of different structural domains, and some of these components can be interchanged to form different forms. Therefore, more forms and derivatives will be created, and some have already been created.
The huge diversity of forms leads to a question: do we really need so many forms, and if so, why? A big reason for so many available structural forms is the strong assembly potential of this modular structure domain diversity. In many cases, these modular domains are like building blocks, and different construction methods produce ever-changing structures.
Therefore, antibody engineering has always been an interesting field, worthy of researchers setting in the biopharmaceutical industry, as well as academic and government institutions. After more than 20 years of development, as long as certain rules are followed, success can now be almost guaranteed. Available (or unavailable) Free Operation (FTO) or Intellectual Property (IP) space, restrictions on certain formats, and the desire to protect their own development may be additional drivers of the explosion of bispecific antibody formats.
These and other factors initially led to some doubts about the necessity of this diversity. “Format Zoo” has now been equated with a playground by a considerable number of designers. We admit that zoos and playgrounds have similar appeals to many young, active, open and exploring humans, and we don’t think this is a bad thing!
At the same time, reality shows that having diverse forms is not only a manifestation of designer wisdom, but also a necessary and a key driving factor for the progress of bispecific antibody therapy. More and more genetically engineered bispecific antibodies are currently in the clinical development stage, some are in the late stage and some are already available as drugs (Table 3).
These bispecific antibodies exhibit very different forms, some are large and contain constant regions, and some are small and do not have constant regions. In fact, most major forms of clusters are composed of one or more molecules that have entered the clinic. Obviously, even without FTO or IP restrictions or competitive/strategic challenges, “one size fits all” cannot be applied to too many applications with desired functions and bispecific antibodies.
Format diversity is required for bispecific antibody applications
Due to the diversity of the bispecific antibodies that need to be produced and the variability of the desired characteristics, it is impossible to have’one optimal format’ for most desired molecular combinations. The expected curative effect (definition of product function and behavior) required by different target products requires various forms of molecular structure, including molecular size, arrangement order, price, flexibility, geometric structure and other aspects. The combination of the product and the binding molecule should also consider the final distribution and pharmacokinetics of the drug.
In order to achieve bispecificity, factors that need to be considered include the special arrangement or size of different target antigens, and in some cases, the density of antigens on the cell surface. This determines which forms can achieve bivalent, trivalent or multivalent binding to one or two antigens simultaneously or mutually exclusive. Small format changes, such as small changes in link length or structure or “switching” of domains, may be key determinants of functionality. Some design parameters can be derived from the structural model. However, in many cases, the function of generating and comparing different formats must be used to determine the appropriate format.
Distribution and pharmacokinetic behavior are other important parameters directly affected by the choice of bispecific antibody format. When a longer half-life is required, the molecular structure design needs to be >50KDa to avoid being cleared by the kidney, and other aspects are designed to ensure that the antibody can be recycled through FcRn. FcRn-mediated recycling is the basic feature of Fc bispecific antibodies (ensure that there are no mutations in the FcRn binding site). Another strategy is to introduce serum albumin to increase FcRn-mediated recycling. Low-molecular-weight bispecific antibodies are used for those that require rapid onset and rapid distribution. Or hope to strictly control its level to choose to quickly reduce the level.
Identifying bispecific antibodies with expected functions is only the first step in drug development
Although format diversity is a prerequisite for bispecific antibodies for different functions, we want to emphasize that in real life, not all zoo members can easily handle it. Some look good, but actually perform poorly.
For drug development, molecules and formats need to be mass-produced in a reproducible manner, preferably with established or similar methods in high-volume production. The more complex the composition structure, the more extensive optimization of the expression system is often required. It must be particularly pointed out that the stability and yield of the expression system are not the only factors that need to be considered. In fact, the composition of the bispecific antibody and the presence or absence of unwanted by-products are of equal (or greater) importance.
In addition to being suitable for production and upstream and downstream processing, bispecific antibodies also need to be clearly defined, stable and overall “good performance” to become drugs. Many of these parameters are discussed under the term “developability”.
Bispecific antibodies that meet the developability criteria will be stable (for example, against thermal denaturation), have a lower tendency to aggregate, a lower tendency to accumulate chemical deviations, and (depending on the method of application) preferentially able to be at high concentrations It is formulated without viscosity problems.
Therefore, designing, optimizing, and characterizing bispecific antibodies with the desired specificity and functionality is only the beginning of a long process. Converting these molecules into drugs is a difficult task. However, this part is the key. Without this part, bispecific antibodies can only be foreign members in the zoo, not drugs.
(source:internet, reference only)
Disclaimer of medicaltrend.org



